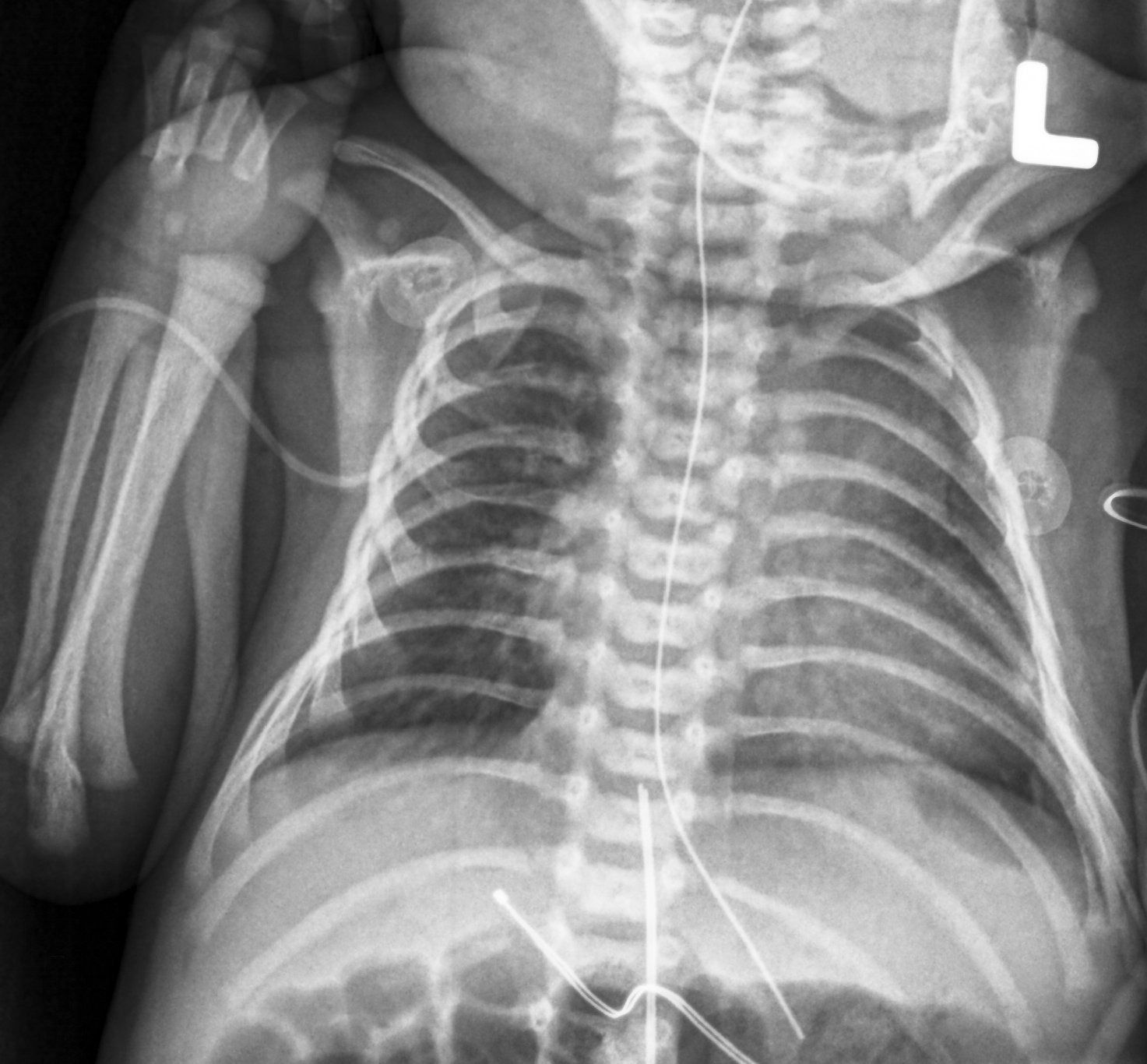
January 26, 2018 — Most people have had an X-ray taken at some time during their lives — perhaps checking for a possible broken bone or during a visit to the dentist. X-ray exams provide important information to physicians about how to treat their patients. However, X-rays use ionizing radiation, and these imaging exams must be carefully and judiciously used on pediatric patients.
In a new guidance, the U.S. Food and Drug Administration’s (FDA) Center for Devices and Radiological Health (CDRH) recommends that medical X-ray imaging exams be optimized to use the lowest radiation dose needed. These exams, which include computed tomography (CT), fluoroscopy, dental and conventional X-rays, should be performed on children and younger patients only when the healthcare provider believes they are necessary to answer a clinical question or to guide treatment.
While the level of risk from the radiation associated with X-rays is small, especially when compared with the benefits of an accurate diagnosis, healthcare professionals must be especially sensitive to their appropriate use in children. Pediatric patients generally require less radiation than adults to obtain a quality image from an X-ray exam, so doctors must take extra care to “child size” the radiation dose.
The level of ionizing radiation from X-ray imaging is generally very low, but can contribute to an increased risk of cancer. Because children have longer expected lifetimes ahead of them for potential effects to appear and the risk for cancer is not fully understood, it is important to use the lowest radiation dose necessary to provide a diagnostic exam, according to the agency.
The FDA is committed to protecting the health of children by providing guidance to manufacturers and users of imaging devices to help lower the exposure to radiation from X-ray exams. The FDA has regulatory oversight of X-ray imaging devices and the companies that make them. To better address radiation safety concerns, the FDA has been encouraging both equipment improvements and better user information.
The FDA also promotes the adoption of improved radiation safety guidelines by professional organizations for both facilities and personnel.
Recommendations
In the new guidance, FDA recommends that medical X-ray imaging exams be optimized to use the lowest radiation dose needed. The FDA defines the pediatric population as birth through 21 years old. However, the optimization of image quality and radiation dose in X-ray imaging depends more on a patient’s size than their age. Smaller patients require less radiation to obtain a medically useful image. Technically, the patient’s body thickness (the distance an X-ray travels through the body to create the image) is the most important consideration when “child-sizing” an image protocol, the agency said.
Unnecessary radiation exposure during medical procedures should be avoided. However, X-rays and CT scans should never be withheld from a child or adult who has a medical condition where the exam could provide important healthcare information that may aid in the diagnosis or treatment of a serious or even life-threatening illness.
What Parents Can Do
The FDA encourages parents and caregivers to talk to their child’s healthcare provider about X-rays and suggests:
- Keeping track of their child's medical-imaging histories;
- Asking the referring physician about the benefits and risks of imaging procedures, such as: How will the exam improve my child's health care? Are there alternative exams to X-rays that are equally useful?; and
- Asking the imaging facility: How does the facility use reduced radiation techniques for children? Is there any advanced preparation necessary?
Patients can report any adverse events to the FDA here.
The Role of Healthcare Professionals
The FDA guidance said that healthcare professionals are responsible for ensuring there is justification for all X-ray imaging exams performed on pediatric patients. They should also consider whether another type of imaging exam that does not expose the patient to ionizing radiation, such as ultrasound or magnetic resonance imaging (MRI), could be used to obtain the same result.
The FDA encourages healthcare professionals and hospital administrators to refer to guidelines and instructions provided at the agency’s Pediatric X-ray Imaging web portal.
For more information: www.fda.gov
Related Pediatric Imaging Content
FDA Issues Final Guidance on Pediatric X-ray Imaging Device Notifications
FDA Approves Guerbet's Dotarem for Pediatric Patients Younger Than Two Years

 February 10, 2026
February 10, 2026