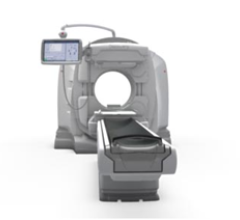
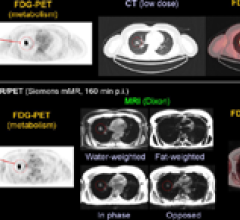
June 10, 2011 – The U.S. Food and Drug Administration (FDA) granted Siemens Healthcare 510(k) clearance for the Biograph mMR, the first system worldwide to enable simultaneous whole-body acquisition of data from magnetic resonance (MR) and positron emission tomography (PET).
June 10, 2011 – Viztek debuted an exam turnaround time (TAT) management feature for its Opal-RAD PACS. Designed to streamline the workflow of teleradiology practices, the feature prioritizes exams based on a client’s contracted time to perform study reading. The feature uses a color-based system to notify radiologists of the remaining time before a scheduled reading must be completed. After users input reading time parameters for each facility, worklist population and priority level is fully automatic.
June 10, 2011 – Dilon Diagnostics introduced the U.S. Food and Drug Administration (FDA)-cleared Dilon 6800 Acella gamma camera system this week at the Society of Nuclear Medicine (SNM) annual meeting in San Antonio, Texas. To complement the Dilon 6800 standard field-of-view imaging system, the new camera will feature a much larger molecular breast imaging (MBI), which will make Dilon the first company in the industry to offer customers a choice in detector sizes.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
June 10, 2011 — At the Society of Nuclear Medicine’s 58th Annual Meeting in San Antonio, June 4-8, Cardinal Health previewed The Center for the Advancement of Molecular Imaging (The Center), its new, first-of-its-kind innovation laboratory, which it plans to open in July 2011.
June 10, 2011 – 3DISC has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its line of FireCR Medical Scanners and QuantorMed Imaging Software in the United States. The FireCR Scanners can be quickly upgraded to higher speeds with a simple smart card update, giving users flexibility to start with an entry?level scanner and increase productivity as their needs change.
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
June 8, 2011 – The U.S. Food and Drug Administration (FDA) has released its list of pre-market approval (PMA) and 510(k) decisions for new or enhanced medical devices from March 2011. The list includes all FDA PMAs , product development protocols (PDP), supplement and notice decisions. This list is generated on a monthly basis.
While most women understand the importance of health screenings, an estimated 72 million have missed or postponed a ...
June 8, 2011 - Brainlab announced that it has entered into a definitive agreement to acquire Voyant Health.
June 8, 2011 – GE Healthcare is offering a new fully upgradeable SPECT technology that allows patient dose as low as 50 percent of those of standard nuclear medicine scanning protocols, or the potential for patients to spend significantly less time on the table during exams, all without compromising image quality.
June 8, 2011 – Rep. Ed Whitfield, R-Ky., has introduced a bill in the House of Representatives that will set federal minimum education and certification standards in the Medicare program for the technical personnel providing, planning and delivering all medical imaging examinations and radiation therapy treatments.
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
June 8, 2011 — GE Healthcare announced the Optima PET/CT 560, which offers efficiency and investment protection with the capability to grow with the future needs of a clinical practice.
June 8, 2011 – Research introduced at the Society of Nuclear Medicine's 58th annual meeting this week may lead to much-needed cardiovascular disease screening for diabetic patients at risk of ischemic heart disease. The disorder is marked by significantly reduced blood flow in the heart. Ischemia of the myocardium can signal diminished oxygenation of the heart tissue and trigger a heart attack if left untreated.
June 8, 2011 – The Society of Nuclear Medicine’s (SNM) 2011 Image of the Year illustrates the ability of positron emission tomography (PET)/computed tomography (CT) scans to identify abnormal bone reaction in patients who have received spinal fixation hardware implants. Researchers selected this image from more than 1,800 studies presented over the course of four days during SNM’s 58th annual meeting in San Antonio, Texas.
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
June 8, 2011 – “Jomo,” the Cincinnati Zoo & Botanical Garden’s 19-year-old silverback, western lowland gorilla underwent a cardiac ultrasound as part of a preventative study. A team of human cardiologists, technicians and corporate partners made the house call to the Cincinnati Zoo’s Gorilla World exhibit recently to perform the echocardiogram to identify any problems.
June 8, 2011 - Double Black Imaging highlighted several new products this week at the Society of Imaging Informatics in Medicine (SIIM) 2011 meeting.
June 8, 2011 - Double Black Imaging (DBI) released their X-Series of self-calibrating LCDs at SIIM.
June 7, 2011 - Digirad and Dilon Diagnostics announced that Digirad has contributed advanced photodetector technology for use in Dilon’s newest gamma camera, the U.S. Food and Drug Administration(FDA)-cleared Dilon 6800 Acella, via a technology development and OEM agreement. The new camera, which is being debuted at the 2011 Society of Nuclear Medicine Meeting in San Antonio, Texas, features the largest molecular breast imaging platform on the market, making Dilon the first company in the industry to offer customers a choice in detector sizes.
June 7, 2011 — Research presented at SNM’s 58th annual meeting is taking targeted molecular imaging to a new level by combining two commonly used imaging agents into one molecular imaging procedure. The combination of these agents creates a comprehensive examination of the extent of cancer spread within a variety of organ systems in the body.
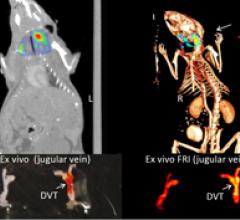
June 7, 2011 — Research presented at the Society of Nuclear Medicine's annual meeting showed a novel optical imaging technique called near-infrared fluorescence (NIRF), which can image dangerous blood clots hiding inside elusive veins. The imaging technique uses light energy with a newly synthesized imaging agent to glean information about cells and tissues. The agent uses a biomarker that seeks out fibrin peptide that is actively involved in the formation of clots.
June 7, 2011 – This year at SNM 2011, Siemens Healthcare introduced Biograph mCT 20 Excel, on display for the first time in a nuclear medicine-focused meeting. Biograph mCT 20 Excel is the latest addition to the Biograph family of PET/CT imagers. This hybrid system offers imaging performance at a lower cost.
June 7, 2011 – Novarad introduces NovaCardio, a fully integrated CPACS image and information management system that offers fast and efficient clinical workflow, powerful features, and accessible anywhere viewing and reporting.


 June 10, 2011
June 10, 2011