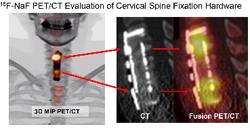
June 8, 2011 – The Society of Nuclear Medicine’s (SNM) 2011 Image of the Year illustrates the ability of positron emission tomography (PET)/computed tomography (CT) scans to identify abnormal bone reaction in patients who have received spinal fixation hardware implants. Researchers selected this image from more than 1,800 studies presented over the course of four days during SNM’s 58th annual meeting in San Antonio, Texas.
Each year, SNM chooses an image that exemplifies the most cutting-edge molecular imaging research today and demonstrates the ability of molecular imaging to detect and diagnose disease and help select the most appropriate therapy. “This year’s image demonstrates the clinical utility of PET/CT using the newly approved PET 18F sodium fluoride bone imaging agent to correctly pinpoint the cause of recurrent pain after surgical placement of spinal fixation hardware,” said Peter Herscovitch, M.D., chair of SNM’s Scientific Program Committee.
Andrew Quon, M.D., assistant professor of radiology and chief of clinical PET/CT for the molecular imaging program at Stanford University, Stanford, Calif., and lead author of the study, further noted, “The NaF PET/CT image shows the potential of molecular imaging for helping patients with a very common orthopedic problem, and it is available to patients right now. It's very exciting because it reminds us that the future of molecular imaging lies not just in cancer imaging but in a wide range of disease processes beyond oncologic applications.”
Serious spinal instability and disease often necessitate the implantation of hardware, such as plates, cages, rods and screws, or bone grafts to support the spine. There are many reasons why patients experience pain after initial surgery, including hardware failure and infection, or both. Determining the source of pain can be difficult, especially when patients have complex medical histories. In this study, a combination of PET/CT and 18F NaF, an injected radiotracer that uses sodium fluoride to target “hot spots” or areas of high bone turnover and inflammation during imaging, was used to evaluate patients with back pain after spinal surgery. This form of molecular imaging was shown to be highly accurate in determining the culprit of patient’s chronic pain by highlighting both the structure of the bone and the physiological processes involved in inflammation, an indication of injury and infection.
For this prospective study, 20 patients presenting with spinal pain were evaluated with PET/CT using 18F NaF at least eight months after surgery. A total of 24 bone or tissue abnormalities were found in 17 of the 20 subjects. Of the original 20 patients, 12 received exploratory surgery and four participants received local anesthetic nerve blockade, a common and minimally invasive treatment that numbs the affected nerve, providing short-term pain management as an alternative to surgery. The research indicates that 18F NaF PET/CT is highly effective for the evaluation of pain after spinal surgery. In more than 85 percent of cases, this form of molecular imaging was able to identify the exact source of patient’s pain.
For more information: www.snm.org


 February 03, 2026
February 03, 2026 









