January 3, 2018 — medQ Inc. recently announced the launch of its Q/ris MIPS Reporting module. This module is designed to ...
The U.S. Food and Drug Administration (FDA) issued the final version of the guidance, “Technical Considerations for Additive Manufactured Medical Devices." Additive manufacturing (AM), the broad category of manufacturing encompassing 3-dimensional (3-D) printing, is an emerging technology. This guidance is not intended to introduce new policy, but rather outlines the Agency’s current thinking about the technical aspects associated with AM processes, and provides manufacturers with recommendations for device design, manufacturing and testing considerations for use when developing devices that include at least one additively manufactured component or additively fabricated step.
Chagas disease (ChD), an infectious parasitic disease transmitted primarily by triatomine insects, has become a significant public health problem in recent decades. While many ChD patients suffer only minor symptoms, 20-30 percent develop chronic heart disease (ChHD); the variety of possible abnormalities caused by ChHD requires understanding of how different imaging modalities can assist in diagnosis and treatment decisions. A new document, Recommendations for Multimodality Cardiac Imaging in Patients with Chagas Disease: A Report from the American Society of Echocardiography in Collaboration With the InterAmerican Association of Echocardiography (ECOSIAC) and the Cardiovascular Imaging Department of the Brazilian Society of Cardiology (DIC-SBC), aims to provide the first comprehensive guidance for how best to use the multiple cardiac diagnostic tools to assess and monitor the growing number of patients with these Chagas-induced heart problems.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Philips and Arizona-based Banner Health recently announced they have extended their multi-year partnership to include adoption of Philips' PerformanceBridge Practice, helping Banner to accelerate its clinical transformation in radiology and further deliver on its goal of improving care for patients. PerformanceBridge Practice is a part of the Philips PerformanceBridge portfolio, a suite of operational performance improvement software and services that assist radiology departments in enhancing productivity, improving the patient and staff experience, while delivering better value-based care. Philips and Banner will work collaboratively to pinpoint improvement opportunities, and a dedicated Philips Solution Advisor will help Banner to develop solutions that address areas for improvement. Banner will roll out PerformanceBridge Practice in all 28 of its radiology departments in a two-phased approach, starting with their 16 Arizona and academic sites, then expanding to its 12 radiology departments in five other states.
Information from brain magnetic resonance images (MRIs) can help identify people with attention deficit hyperactivity disorder (ADHD) and distinguish among subtypes of the condition, according to a study appearing online in the journal Radiology.
Sixty million women undergo regular screening mammography in the United States, but even in the digital age, it is ...
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
MEDraysintell recently downgraded its projection for proton therapy rooms expected to be operational in 2030 from 1,200 to 900. The company said this still represents a 12 percent annual growth from 2017 to 2030, and the need to open an average of more than 50 proton therapy treatment rooms every year from 2018 to 2030.
Seno Medical Instruments Inc. (Seno Medical) recently reported data from their clinical study demonstrating that its Imagio opto-acoustic (OA) breast imaging system achieved significant differentiation among prognostic subtypes of breast cancer (PSBC). These prognostic subtypes provide important information about the genes that each patient's cancer expresses. The data were presented during a poster presentation at the San Antonio Breast Cancer Symposium, Dec. 5-9 in San Antonio, Texas.
iCAD Inc. announced that its Xoft Axxent Electronic Brachytherapy (eBx) System is now available at two California-based centers from The US Oncology Network – Santa Clarita Radiation Therapy Center and Sherman Oaks Radiation Therapy Center. Local patients with non-melanoma skin cancer (NMSC), including individuals precluded from surgery or those seeking a non-invasive option, will now have access to a proven alternative.
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
IBA (Ion Beam Applications S.A.) announced in November the acceptance and beam model validation of the first Varian Halcyon machine in Europe to go clinical, at the hospital UZ Leuven in Belgium, using IBA Dosimetry’s Blue Phantom water phantom system.
January 2, 2018 — A new study found women who had received chemotherapy and/or radiation therapy to treat breast cancer ...
December 29, 2017 — Radiologists can enhance the quality and effectiveness of care with the newest release of the ACR ...
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
Migraine sufferers have significantly higher sodium concentrations in their cerebrospinal fluid than people without the condition, according to the first study to use sodium magnetic resonance imaging (MRI) to look at migraine patients. The findings were presented at the annual meeting of the Radiological Society of North America (RSNA), Nov. 26-Dec. 1 in Chicago.
December 28, 2017 — At the 2017 Radiological Society of North America (RSNA) Annual Meeting, Nov. 26-Dec. 1 in Chicago ...
December 28, 2017 — Double Black Imaging and their Image Systems Division are releasing their Gemini Series 6MP and 8MP ...
In the December featured basic science article in The Journal of Nuclear Medicine, Belgian researchers report on the first large-scale longitudinal imaging study to evaluate BACE1 inhibition with micro-PET (positron emission tomography) in mouse models of Alzheimer’s disease. PET imaging has been established as an excellent identifier of the amyloid plaque and tau tangles that characterize Alzheimer’s disease. Now it is proving to be an effective way to gauge treatment effectiveness.
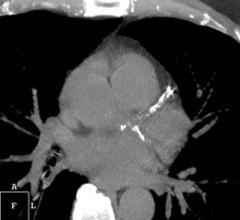
HeartFlow Inc. announced that Health Care Service Corp. (HCSC), which operates Blue Cross and Blue Shield plans in five states, has issued a medical policy for the HeartFlow FFRct Analysis, a non-invasive technology that helps clinicians diagnose and treat patients with suspected coronary artery disease (CAD). HCSC — which operates Blue Cross Blue Shield plans in Illinois, Montana, New Mexico, Oklahoma and Texas — has determined that the use of noninvasive fractional flow reserve (FFR) following a positive coronary computed tomography (CT) angiogram may be considered medically necessary to guide decisions about the use of invasive coronary angiography in patients with stable chest pain at intermediate risk of CAD.
Philips recently announced that it acquired VitalHealth, a provider of cloud-based population health management solutions for the delivery of personalized care outside of the hospital, for example, in regional care networks. Headquartered in the Netherlands, VitalHealth’s products and services are being used by more than 100 healthcare networks in various countries including the United States, India, China, Sweden, Germany, Belgium and the Netherlands. The company was founded in 2006 by Mayo Clinic (U.S.) and Noaber Foundation (the Netherlands) and employs approximately 200 employees. Financial details of the transaction were not disclosed.
The Imaging Technology News (ITN) website had another record year with more than 1.25 million page views in 2017. Rad ...
The U.S. Food and Drug Administration (FDA) has cleared the new GammaPod noninvasive stereotactic radiotherapy system intended for use in treating cancer in breast tissue.

 January 03, 2018
January 03, 2018