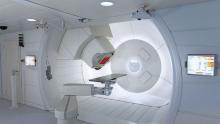
As one of the more precise methods of delivering radiation therapy for cancer treatment, proton therapy has grown at a rapid pace the last several years. Considered experimental not too long ago and used primarily in research settings, clinicians across the globe are rapidly coming to accept the utility of proton therapy for numerous indications — a list that many expect to continue to grow rapidly.
If you enjoy this content, please share it with a colleague
- Read more about Proton Therapy Continues Rapid Growth
- Log in or register to post comments