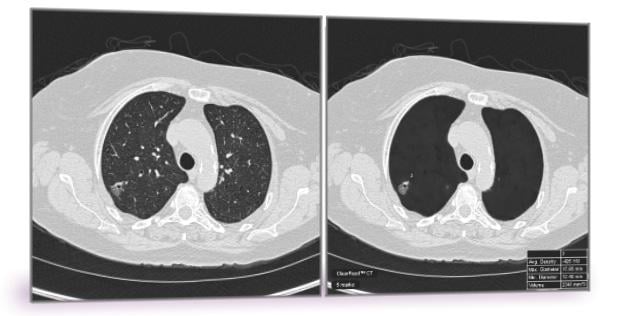
June 14, 2018 — Riverain Technologies announced that the United States Patent and Trademark Office (USPTO) has awarded the company a broad patent for its artificial intelligence (AI) computed tomography (CT) technology. The technology in question forms a vessel suppressed, chest CT series using deep learning and synthetic data modeling. The company said the patent holds promise for improving the detection and diagnosis of lung cancer at an earlier stage while making clinicians more efficient, a first for computer-aided detection (CAD).
Riverain has been issued U.S. Patent 9,990,743 for its methodology to selectively remove anatomical structures from CT scans to optimize reading accuracy and efficiency for lung cancer detection. The technology is used in the company’s premier software, ClearRead CT. Riverain said ClearRead CT is proven to lead to more accurate and efficient detection of lung nodules, the early indicator of lung cancer. A range of possible applications exists within other imaging modalities including magnetic resonance imaging (MRI), positron emission tomography (PET), full field digital mammography (FFDM) and tomosynthesis, where normal structures interfere with the detection and diagnosis of disease.
For more information: www.riveraintech.com
Related Content
Riverain Technologies Earns FDA Approval for ClearRead CT Software
VIDEO: Technology Report: Artificial Intelligence 2017
PODCAST: Imaging Smack Down at SIIM: AI Won’t Soon Replace Radiologists, With Eliot Siegel


 February 13, 2026
February 13, 2026 









