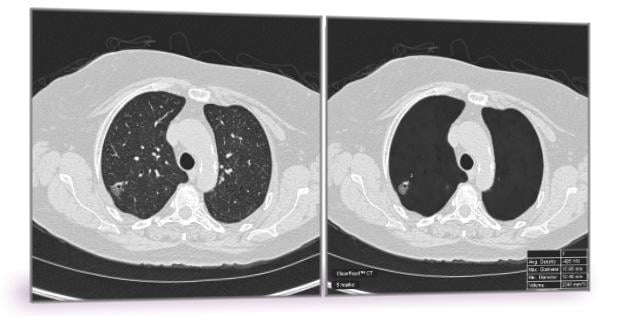
September 20, 2016 — Riverain Technologies announced that its ClearRead CT, a breakthrough lung nodule detection application, has received clearance from the U.S. Food and Drug Administration (FDA).
ClearRead CT is the only FDA-cleared device to support concurrent reading, allowing for faster reading with proven superior automatic nodule detection performance for all primary nodule types, including solid, sub-solid and ground glass nodules.
ClearRead CT, powered by acquisition normalization technology, provides an enterprise-ready solution for the entire healthcare network. The technology seamlessly processes computed tomography (CT) scans from a wide range of manufacturers and acquisition protocols, and quickly installs into the clinical environment without the need for new hardware or customized tuning to specific devices or protocols.
ClearRead CT was evaluated in a multi-reader, multi-case clinical trial and showed radiologists achieved a 29 percent reduction in missed actionable nodules, while reducing reading time by 26 percent.
The software is comprised of two tools: ClearRead CT | Vessel Suppress and ClearRead CT | Detect. Deep learning enables the vessel suppression technology to assist both machine and humans in the detection and characterization of all primary nodule types, allowing for previously unattained nodule detection performance.
ClearRead CT is available for sale in the United States, Canada and Europe.
For more information: www.riveraintech.com


 March 05, 2026
March 05, 2026 









