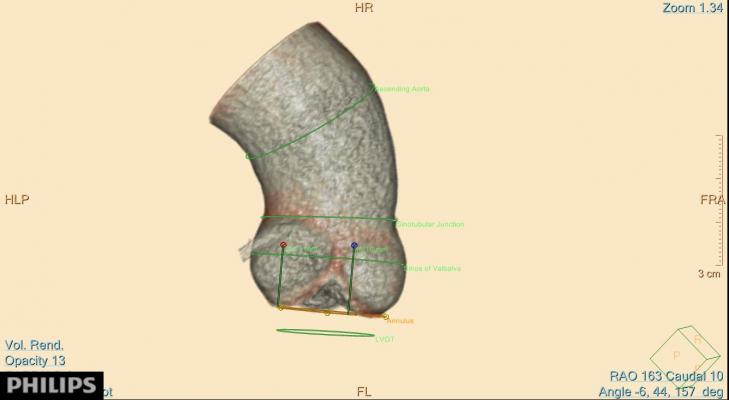
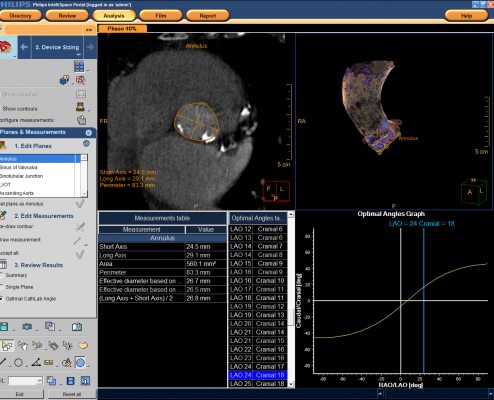
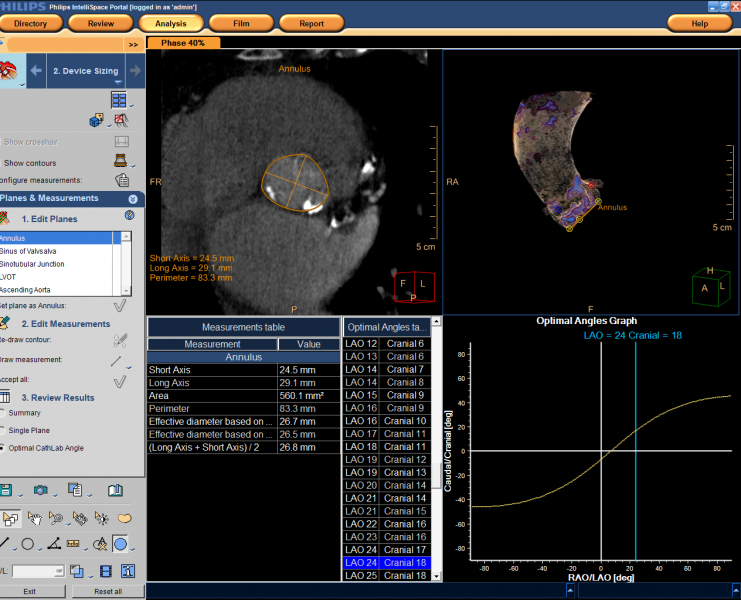
August 27, 2014 — Philips Healthcare announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its precision planning application for transcatheter aortic valve implantation (TAVI) treatments. Through 3-D imaging, the Philips TAVI application provides interventionalists with pre-procedural, high-precision positioning to treat aortic stenosis ailments.
The Philips TAVI planning application is available as part of Philips IntelliSpace Portal 6, the company's advanced visualization and analysis solution that allows clinicians to access and analyze patient imaging and data virtually anywhere, at any time. The application features a comprehensive measurement package to accommodate virtually all types of TAVI devices.
"As our population ages, minimally invasive TAVI procedures are becoming increasingly popular in the United States, since they provide a non-surgical option for those patients who might have once been considered too high-risk for heart surgery," said Gene Saragnese, CEO, Imaging Systems, Philips Healthcare. "Treating cardiac conditions requires intense precision, and our TAVI planning application delivers a solution for aortic device placement to help improve patient care."
Through advanced computed tomography (CT) imaging, the TAVI planning application provides planes and panel measurements for precisely placing TAVI devices to manage the risk of under- or oversizing of a TAVI device. It renders images into a 3-D heart model to allow interventionalists to orient the device and address less-than-optimal patient cases.
The TAVI technology received FDA approval in 2011. A minimally invasive procedure, TAVI became a major breakthrough option for high-risk patients who were deemed non-surgical candidates. This procedure can reduce mortality by 60 percent as compared to conventional surgeries.
The Philips TAVI application will be on display at the upcoming European Society of Cardiology (ESC) Congress 2014.
For more information: www.usa.philips.com/healthcare


 February 09, 2026
February 09, 2026 









