May 22, 2019 — Research has demonstrated use of Volpara Solutions' VolparaDensity software in combination with the Tyrer-Cuzick Breast Cancer Risk Evaluation Tool improves breast cancer risk stratification. The research suggests VolparaDensity could be used to help guide personalized medicine through risk-adapted screening.
The study, "A case-control study to add volumetric or clinical mammographic density into the Tyrer-Cuzick breast cancer risk model," was published in the May 11 issue of Journal of Breast Imaging, the official journal of the Society of Breast Imaging.
The paper used visual BI-RADS (Breast Imaging-Reporting and Data System) 4th Edition assessments from breast imaging specialists at the University of Virginia (UVA). It included 474 women who were diagnosed with invasive breast cancer alongside 2,243 controls. The results demonstrated that the addition of volumetric and visual mammographic density measures to classical risk factors improves risk stratification, increasing the number of women accurately identified at high and lower risk of breast cancer. The Tyrer-Cuzick Tool, one of the most complete and widely used risk models, now accepts VolparaDensity volumetric breast density assessment as a validated breast density input in Version 8.
Based on the study results, researchers concluded that risk could be used to guide precision medicine through risk-adapted screening and prevention strategies. Further, they noted in the discussion that "volumetric breast density assessment has some practical advantages because it is fully automated and has excellent agreement with 3-D breast MRI [magnetic resonance imaging]."
Jack Cuzick, Ph.D., John Snow Professor of Epidemiology, director, Wolfson Institute of Preventive Medicine, and head, Centre for Cancer Prevention, stated, "Including breast density into risk models makes sense. Breast density is a strong risk factor for breast cancer, and it is largely independent of all other known risk factors. Breast density contributes as much information as all other factors combined, nearly doubling the predictive value of the risk model. Screening programs need to take this into consideration in order to develop a personalized breast care plan for each woman."
Eric J. Kraemer, M.D., radiologist at Reno Diagnostic Centers, stated, "Incorporating an objective density measure and the Tyrer-Cuzick risk model has allowed us to better personalize screening for all of our patients. Patients with a lifetime risk of greater than 20 percent are recommended for mammography and MRI screening. The personalized plan for patients with dense breasts but with lower risk includes mammography and automated breast ultrasound (ABUS). For patients that do not have dense breasts and that are at low risk of developing breast cancer, mammography continues to be the gold standard in breast cancer screening. Developing a personalized breast care for each woman depending on her specific risk factors means that we can do a better job at detecting cancer earlier, ensuring that lives are saved."
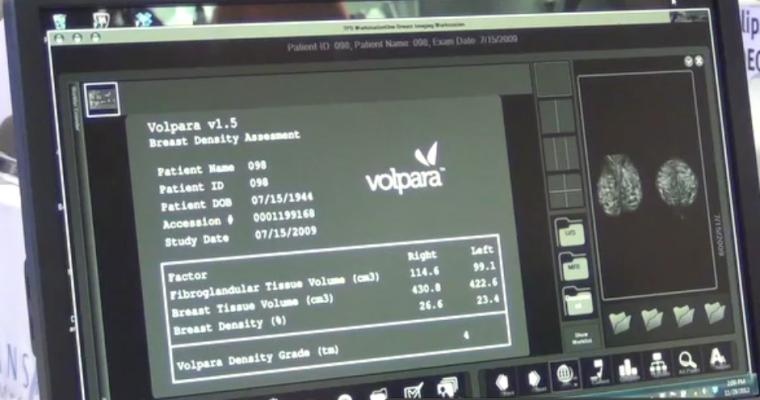
As one of the clinical applications within VolparaEnterprise, the VolparaDensity clinical application provides radiologists with automated, objective volumetric breast density assessments and a breast density category shown to correlate to BI-RADS 4th and 5th Editions. It is CE-marked and cleared by the U.S. Food and Drug Administration (FDA), Health Canada and the TGA.
For more information: www.volparasolutions.com
Reference
1. Brentnall A.R., Cohn W.F., Knaus W.A., et al. A Case-Control Study to Add Volumetric or Clinical Mammographic Density into the Tyrer-Cuzick Breast Cancer Risk Model. Journal of Breast Imaging, May 11, 2019. doi.org/10.1093/jbi/wbz006


 February 05, 2026
February 05, 2026 









