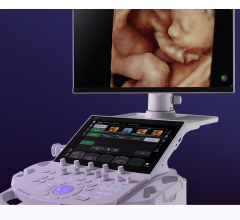
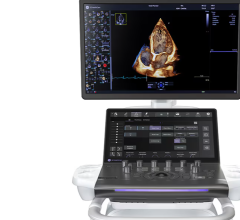
October 5, 2009 - The FDA has given Sonosite 510(k) clearance for its new NanoMaxx ultrasound system, a new portable, 6-lb. ultrasound with a one button design.
Based on SonoSite’s 4th generation Turbo technology, the 6-lb. NanoMaxx system is the latest addition to SonoSite’s suite of specialized products for point-of-care visualization in medicine. Complete with streamlined one button optimization technology, a touch screen user interface and SonoSite’s 5-year warranty, the NanoMaxx system suits both hospital and office markets and is designed to provide healthcare professionals with improved patient safety practices, expanded workflow capabilities and exam room flexibility.
The new ultra portable and one button design of the NanoMaxx system makes high quality ultrasound available to a much broader range of clinicians. The system incorporates SonoSite’s advanced proprietary imaging algorithms, including SonoMB and SonoAdapt to deliver superior image quality in a lightweight, rugged form factor.
With a touch screen that responds easily to the tap of a finger, and one button optimization, clinicians can readily acquire high resolution images to increase clinical productivity at the point-of-care. A system boot-up time of less than 20 seconds and long battery life further enhance workflow when using the NanoMaxx system.
At introduction, the NanoMaxx tool is available with a complement of five transducers to support a wide range of examinations and procedures including thoracic assessment for pathology, vascular access, needle aspirations and injections, as well as abdominal, cardiac, nerve, OB/Gyn, musculoskeletal, small parts and vascular scanning. The clinical capabilities of the NanoMaxx will help eliminate the risk and cost of transporting patients to the imaging lab for many examinations and procedures.
The small footprint of the system, along with its space-saving solutions, enables seamless integration with a multitude of exam-room configurations. For convenience and flexibility, the system can be wall mounted, placed on an exam table with kickstand attached, or used from a highly maneuverable stand. Physicians can easily carry the NanoMaxx tool from room-to-room, to a satellite office, to the operating room or to a field site for immediate use.
The NanoMaxx system’s highly integrated architecture and ruggedized design, including the industry’s first elastomeric bumper for extra durability, plus magnesium outer shell, allows it to be used in the most demanding and austere environments. Proving its reliability, the NanoMaxx system and its transducers successfully passed SonoSite’s standard three foot durability drop test.
In addition, to further reduce the risk of infection, the NanoMaxx system’s fluid-resistant user interface makes the system easy to clean and disinfect, helping to address the growing concern over infection control in the medical community.
Affordable and Easily Accessible with Web-Based Purchasing Program
Affordably priced, the NanoMaxx system and its transducers include SonoSite’s industry-leading 5-year warranty, to provide the lowest cost of ownership in ultrasound.
Also, SonoSite’s One-to-One Purchase Program further extends the cost saving advantages of the NanoMaxx ultrasound tool by replacing or complementing the traditional on-site, pre-sale demonstration with a personal phone consultation and webcast demonstration provided by a professional clinical sales consultant. Visit the NanoMaxx ultrasound tool website to learn more about SonoSite’s newest system and to experience a personal, interactive product demo. In addition, Apple iPhone and iPod mobile device users can view a video of the NanoMaxx system on SonoSite’s new SonoAccess application.
For more information: www.sonosite.com


 February 05, 2026
February 05, 2026