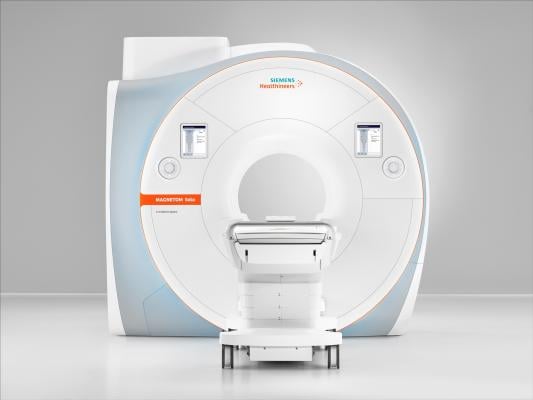
March 4, 2019 — South Texas Radiology Imaging Centers, San Antonio, recently became the first healthcare institution in the United States to install the Magnetom Sola 1.5 Tesla (1.5T), 70-cm magnetic resonance imaging (MRI) scanner from Siemens Healthineers. The Magnetom Sola features a new magnet design in addition to BioMatrix patient personalization technology.
The system’s BioMatrix technology addresses patients’ anatomical and physiological differences, as well as user variability, to help healthcare providers quickly perform a full range of routine and complex examinations while delivering consistent results for each patient. With this technology, the Magentom Sola can help decrease rescans and increase productivity to improve cost efficiency and the overall patient experience. For example, Select&GO one-touch positioning uses artificial intelligence (AI) to accelerate patient positioning by up to 30 percent compared to laser positioning, helping providers avoid delays due to incorrect positioning.
The new MRI scanner also possesses Turbo Suite, which enables the acceleration of routine scans, including musculoskeletal (MSK) scans, by up to 50 percent. Additionally, Dot (Day optimizing throughput) technology can automate up to 90 percent of exams.
“Given the increasing costs of providing high-quality healthcare while hurdling the challenges of declining reimbursement, we chose a platform that was scalable across our enterprise,” said Gary Woodard, chief executive officer for South Texas Radiology Imaging Centers. “This allows us to manage our protocols while enabling technologists to customize care for each patient through protocol management under the real-time direction of the radiologist. The enterprise-wide Dot technology allows efficient optimization of our protocols, which can then be changed one time and duplicated seamlessly across our fleet of Siemens Healthineers MRI systems.”
“It was really surprising to see the abdominal MRI image quality,” noted Michael Orsi, M.D., body radiologist at South Texas Radiology Imaging Centers. “The difference was very clear from the first exam; in particular, the post-contrast images had virtually no motion artifacts despite the patient being relaxed and free-breathing. The magnet makes imaging our most challenging patients very easy.”
For more information: www.usa.healthcare.siemens.com


 February 13, 2026
February 13, 2026 









