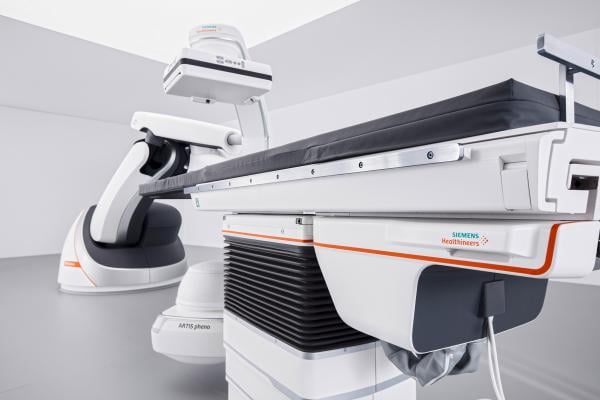
July 5, 2017 — Michigan Medicine recently became the first healthcare institution in the United States to install the Artis pheno, the new robotic C-arm angiography system from Siemens Healthineers. The system is designed to broaden clinical capabilities and expand patient access while combating patient infection.
The Artis pheno robotic C-arm angiography system is designed to be unobtrusively moved in and out of the surgical area. This versatility in movement, coupled with the system’s wider C-arm design, increases the amount of free space and permits greater patient access. The expanded weight capacity and heightened flexibility in table positioning allow clinicians to broaden their spectrum of patient cases and interventional procedures.
The wide array of advanced guidance tools offered on the system aid in the performance of minimally invasive procedures. With tools dedicated to improving visualization and streamlining the procedural workflow, the system is designed to support less invasive patient procedures. Several new features on the Artis pheno were developed to assist hospitals in their efforts to combat infection. Due to the absence of ceiling components, an uninterrupted sterile airflow can be maintained and the system’s sealed, antimicrobial surfaces provide infection resistance in addition to facilitating system cleaning.
For more information: www.usa.healthcare.siemens.com

 September 10, 2025
September 10, 2025 









