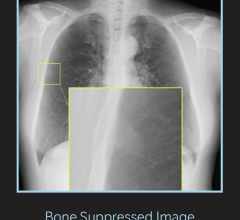
Multix Fusion image courtesy of Siemens Healthcare.
January 30, 2015 — Siemens Healthcare announced that a two-detector version of its established Multix Fusion digital radiography (DR) system has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and is now available in the United States.
The two-detector iteration of the Multix Fusion – a digital imaging system Siemens introduced in 2012 – features digital cassette-sized flat-panel detectors, a large fixed detector in the wall stand, a height-adjustable table with a 660-pound weight capacity and a touchscreen positioned at the overhead tube suspension for exam parameter changes at the patient’s side.
Multix Fusion is designed for customers with high patient throughput who are looking for excellent image quality at low dose. The system’s two detectors offer high positioning flexibility between the table and wall stand, covering routine to advanced applications such as ortho, long leg and full spine studies. The system’s high level of image contrast is the result of its DiamondView Plus on-board post-processing tool, and the automated image processing parameter provides settings based on organ programs.
For more information: www.healthcare.siemens.com

 February 10, 2026
February 10, 2026