December 2, 2016 — Samsung Electronics showcased the GM85 digital radiography (DR) system, which received 510(k) U.S. Food and Drug Administration (FDA) clearance in November, at the Radiological Society of North America (RSNA) 2016 annual meeting, Nov. 27-Dec. 1 in Chicago.
Along with the GM85, Samsung displayed a wide range of medical imaging solutions, from premium mobile digital X-ray to advanced ultrasound systems. Moreover, new clinical research showing how Samsung’s latest ultrasound technology drives better patient care was presented at its RSNA Corporate Symposium session.
On display at Samsung’s booth were the following technologies:
Digital Radiography (DR)
GM85
- Easy navigating with compact design: With 555 mm narrow width and weighing in at a light 349 kg, the GM85 allows easy access around tight spaces, even in elevators. A collapsible column gives users clear visibility when moving the system and broadens access to smaller spaces. Adaptive soft-driving control and front-bumper sensor also help make navigation safer.
- Optimized workflow with enhanced usability: SID (Source to Image Distance) Guide supports detailed device positioning with multiple SID settings. S-Align displays the detector’s angle to the THU (Tube Head Unit) for precise alignment to enhance image quality. Quick-positioning allows handle-free, precise body movement by simple button clicks on the THU and optimizes workflow, enabling users to save time.
- Advanced imaging technology for superior image quality: S-Vue, the advanced imaging engine, enhances image sharpness and clarity. SimGrid provides high-quality images without using a conventional grid, reducing scatter radiation effects. Also, radiographers can lower retake rates as S-Vue eliminates alignment errors that often occur with a conventional grid.
GC85A (Advanced Applications) adds new features to radiography workflows:
- Fast stitching: Upgraded stitching technology reduces process time by 31 percent compared to the original GC85A. Increased process speed means radiographers receive exam images more quickly. Fast stitching helps lessen the burden on patients who find it difficult to stand for an extended period of time and leave the X-ray room faster than before.
- SimGrid: This feature streamlines workflow because it operates without a portable grid, reducing operator wrist overload. SimGrid also eliminates alignment errors that often occur with portable grids, reducing retake rates and increasing patient satisfaction.
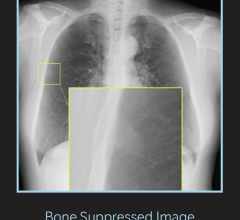
- Bone suppression: Clarity of soft tissue is improved when suppressing the appearance of bones in chest images.
Portable Computed Tomography (CT)
The BodyTom and CereTom portable systems are utilized in a wide range of market applications such as mobile stroke units, brachytherapy suites and proton therapy centers, deep brain stimulation and even veterinarian hospitals.
CereTom has seen more mobile stroke treatment units implement the CT scanner:
- The use of CereTom-equipped mobile stroke units expands to several new cities, including New York City, Chicago and Trenton, N.J.; and
- CereTom was awarded a showcase spot among 14 medical innovations before thousands of hospital executives at Premier Inc.’s Annual Innovation Celebration during the company’s 2016 Breakthroughs Conference and Exhibition June 21 for healthcare providers.
BodyTom expands into new care paths as radiologists find more value:
- The University of Texas MD Anderson Cancer Center in Houston, Texas added BodyTom CT, the world’s first portable, full-body, 32-slice CT scanner to its Proton Therapy Center and is now treating patients at the facility using images from the scanner;
- The University of Wisconsin (UW) Carbone Cancer Center in Madison recently incorporated BodyTom technology into its radiation oncology brachytherapy planning process; and
- Willis-Knighton Cancer Center in Shreveport, La., also added BodyTom® CT to its brachytherapy suite.
Ultrasound
Samsung offered live scanning demos of the RS80A with Prestige at RSNA. Features include:
- CEUS+: On the heels of the recent FDA approval of contrast-enhanced ultrasound for liver lesions in adults and pediatrics, Samsung introduces its latest contrast-enhanced ultrasound imaging, CEUS+. The superb resolution and improved uniformity help users acquire better clarity in the near field. Its automatic brightness-control feature optimizes imaging in real-time, providing users with enough time to diagnose. The CEUS+ also improves the diagnosis of small lesions, and its clear expression of tissue boundaries helps users to achieve interventional procedures with ease and accuracy; and
- S-Detect for Breast [pending FDA 510(k) clearance, not yet available in the U.S.] – Applying data from 10,000 breast exams to ultrasound imaging, S-Detect helps radiologists understand characteristics of breast lesions to determine whether they’re malignant or benign in a few clicks. The S-Detect provides faster and more accurate results by adopting a big-data, deep-learning algorithm in lesion segmentation, characteristic analysis and assessment processes.
For more information: www.samsungmedicalsolution.com

 February 10, 2026
February 10, 2026