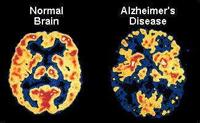
August 2, 2011 – Two new studies published in the August 2011 issue of The Journal of Nuclear Medicine (JNM) provide insight into the potential of positron emission tomography (PET) to differentiate between types of dementia and to identify pharmaceuticals to slow the progress of dementia. With proposed National Institute on Aging (NIA) and the Alzheimer's Association guidelines for detecting Alzheimer's-related brain changes expected in September, these articles give a preview of what may be to come.
Earlier this year, the NIA and the Alzheimer's Association released new criteria and guidelines for the diagnosis of Alzheimer's disease. The new proposed guidelines, available this fall, will offer additional information regarding the development of tests to measure biological changes in the brain, blood or spinal fluid to diagnose Alzheimer's at an earlier stage.
Earlier diagnosis of Alzheimer's disease is the focus of the JNM article "Amyloid Imaging with 18F-Florbetaben in Alzheimer Disease and Other Dementias." In this study, researchers compared cortical amyloid deposition using 18F-florbetaben and PET in 109 controls and subjects with mild cognitive impairment (MCI), frontotemporal lobar degeneration, dementia with Lewy bodies, vascular dementia, Parkinson's disease and Alzheimer's disease.
The results show that 18F-florbetaben performs with the same high accuracy as previously reported with 11C-Pittsburgh Compound B –– the most specific and widely used amyloid imaging agent –– for distinguishing between certain types of neurodegenerative dementia, particularly for diagnosis of Alzheimer's disease from frontotemporal dementia.
"The difference between 11C-Pittsburgh Compound B and 18F-florbetaben is that the 18F-florbetaben has a longer half life and is more affordable, making it appropriate for clinical use," said Christopher Rowe, M.D., FRACP, one of the authors of the study. "This distinction profoundly affects treatment and prognosis and has genetic implications for the family."
In addition to detecting Alzheimer's disease earlier, molecular imaging can also be used in clinical trials to help develop pharmaceuticals to prevent or delay the onset of dementia. This is particularly of importance to patients with MCI who have yet to develop Alzheimer's disease.
"We urgently need tools for conducting drug trials for MCI more efficiently," noted Karl Herholz, M.D., lead author of the study "Evaluation of a Calibrated 18F-FDG PET Score as a Biomarker for Progression of Alzheimer's Disease and Mild Cognitive Impairment." He continued, "Clinical outcome parameters show large variability and little sensitivity to progression at that stage, making these trials extremely costly and cumbersome. By using imaging biomarkers as primary outcome parameters, clinical trials can be performed with smaller sample sizes or shorter trial duration without loss of study power.
The study evaluated a predefined quantitative measure –– a PET score –– that was extracted automatically from 18F-FDG (fludeoxyglucose) PET scans using a sample of controls, patients with MCI and patients with Alzheimer's disease. The PET scores provided a much higher test-retest reliability than standard neuropsychologic test scores (Alzheimer's Disease Assessment Scale-Cognitive and Mini-Mental State Examination) and superior strength for measuring progression, as well as a valid measurement of cognitive impairment. As such, the PET scores can be considered a valid imaging biomarker to monitor the progression of MCI to Alzheimer's disease.
"Prevention of dementia by drugs applied at MCI stage would greatly improve quality of life for patients and reduce costs of dementia care and treatment. Thus, development of such drugs and efficient tools for testing them are extremely important," said Herholz.
Alzheimer's disease is an irreversible, progressive brain disease that slowly destroys memory and thinking skills and, eventually, the ability to carry out the simplest tasks of daily living. Although treatment can slow the progression of the disease and help manage its symptoms, there is no cure for Alzheimer's disease. The Alzheimer's Association estimates that more 5 million people are currently living with the disorder.
For more information: www.jnm.snmjournals.org


 February 13, 2026
February 13, 2026 









