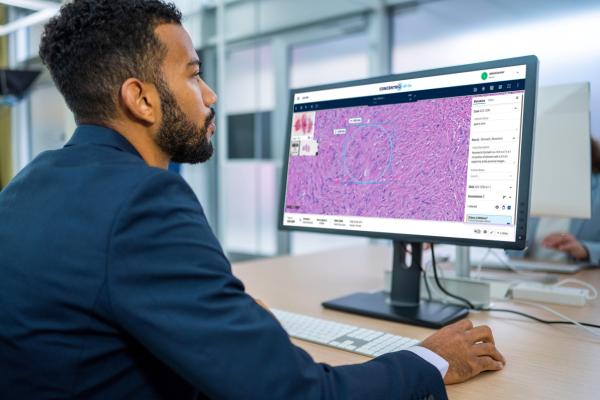
Proscia has announced its receipt of 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Concentriq AP-Dx for the purpose of primary diagnosis. The diagnostic software solution is used for viewing, interpreting, and managing whole slide images, reported the company, noting this marks a significant regulatory milestone advancing the company’s mission to perfect cancer diagnosis. Image courtesy: Proscia
February 12, 2024 — Proscia, a leading provider of digital and computational pathology solutions, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Concentriq AP-Dx. The digital pathology solution was cleared for the purpose of primary diagnosis, reported the company, adding that it is cleared (K230839) for clinical use with the Hamamatsu NanoZoomer S360MD Slide scanner in the United States.
Proscia’s Concentriq AP-Dx is a comprehensive diagnostic software solution that immerses pathologists in an intuitive experience for viewing, interpreting, and managing whole slide images and helps to drive confidence and efficiency gains. It can also streamline collaboration, broadening access to expertise. Concentriq AP-Dx was designed to be used in clinical settings of all sizes, from individual reference laboratories to the largest hospital systems.
In support of its 510(k) clearance, Proscia conducted a multi-site clinical study at PathGroup, South Bend Medical Foundation, and Spectrum Healthcare Partners. The study demonstrated that diagnoses made on Concentriq AP-Dx are non-inferior to traditional glass slide reads. Relative to ground truth data, the difference in major discordance rates for slides read digitally and for slides read using the microscope was -0.1%, which is among the strongest findings of similar publicly available studies.
“This regulatory milestone reflects our tireless commitment to our mission of perfecting cancer diagnosis,” said David West, Proscia’s CEO. West added, “Pathologists are facing more pressure than ever before in the fight against some of humanity’s biggest challenges. With 510(k) clearance, we can help more laboratories improve the pathologist experience and better serve their patients.”
Up to 70% of clinical decisions depend on pathology1. Digital pathology shifts the standard from microscope to high-resolution images each containing over 1 billion pixels that tell the story of a patient’s disease and hold the keys to advancing precision medicine. It is also driving powerful efficiency gains that help laboratories to overcome systemic challenges; hiring for pathologists is near an all-time high2 as the number of new cancer cases per year in the U.S. is expected to cross 2 million in 2024 for the first time ever3.
In addition to its 510(k) clearance, Proscia was the first company to achieve CE-IVDR certification to advance primary diagnosis in the European Union. The company also has a product licensed in Canada and cleared in the United Kingdom among other countries. More than 10,000 pathologists and scientists, including those at top diagnostic laboratories and 14 of the top 20 pharmaceutical companies, trust Proscia’s software, reported the company in its statement.
Reference:
1. NHS England (2017). Digital First: Clinical Transformation Through Pathology Innovation. National Pathology Programme; doi: https://www.england.nhs.uk/wp-content/uploads/2014/02/pathol-dig-first…
2. Klipp, J. (2023). Pathologist Job Openings Remain Near Record High. Laboratory Economics, October 2023. 18(10) p. 10.
3. Collins, S. (2024, January 17). 2024 – First Year The US Expects More than 2M New Cases Of Cancer. The American Cancer Society. Retrieved January 26, 2024, from: https://www.cancer.org/research/acs-research-news/facts-and-figures-202…
More information: www.proscia.com
Related content:
PROSCIA ACHIEVES ISO 27001 SECURITY CERTIFICATION
PROSCIA’S CONCENTRIQ DX ATTAINS CE MARK UNDER IVDR FOR USE IN PRIMARY DIAGNOSIS
PROSCIA INTRODUCES AI-POWERED QUALITY CONTROL TO ACCELERATE DATA-DRIVEN DRUG DEVELOPMENT


 March 04, 2026
March 04, 2026 









