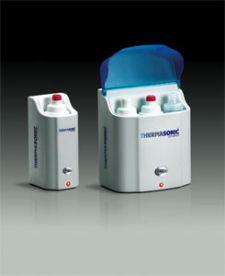
Parker Laboratories, the world-leading manufacturer of ultrasound and electromedical contact media, announces two new THERMASONIC Gel Warmers; a new single bottle gel warmer and a newly designed three-bottle gel warmer.
The three-bottle gel warmer features an adjustable thermostat control and, heat indicator lamp, identifying when the heating element is on or when the desired temperature has been reached, and a illuminated power switch. It warms three dispenser size couplant bottles simultaneously and comes complete with a two- year warranty. The new single bottle gel warmer contains a heat indicator lamp and also comes complete with a warranty. Both units contain hospital grade plug and power cords. The gel warmers allow couplants to be applied at body temperature, promoting patient comfort and receptivity.
THERMASONIC is a durable, self-contained unit, constructed to give years of trouble-free service. THERMASONIC is UL Listed (Underwriters Laboratories, Inc.) as well as CSA Certified (Canadian Standards Association) and are available in 120v and soon to be available in 230v CE certified units.
THERMASONIC Gel Warmer is available through medical supply distributors, or by contacting Parker Laboratories, Inc., 286 Eldridge Road, Fairfield, NJ 07004, USA. Telephone: (973) 276-9500, Toll Free: (800) 631-8888, Fax: (973) 276-9510.


 February 05, 2026
February 05, 2026 









