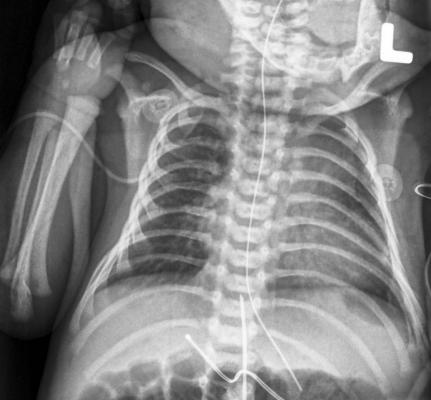
December 17, 2014 (November 20, 2014) – Kubtec announced U.S. Food and Drug Administration (FDA) approval of the KUB 250, a portable low-dose digital X-ray system dedicated to the neonatal intensive care unit (NICU). The unit is compact and lightweight, sporting a 24-inch x 24-inch footprint and weighing 200 pounds.
When imaging high-risk infants in the NICU, it is critical to track subtle changes in pathology while maintaining the focus on low dose. Current digital X-ray systems maintain pixel resolution in the 150-170 micron range, thereby sacrificing high-resolution images in order to keep the radiation dose low. The poor image quality may require facilities to take additional X-rays of the neonate to confirm pathology or PICC line placement, which defeats the purpose of imaging with reduced radiation exposure.
The KUB 250 images with 96-micron resolution and features 750 ms image readout, providing pediatric radiologists with high-quality images at the lowest dose every time. This minimizes radiation dose by as much as 40 percent.
Featuring the smallest footprint in its class, the highly mobile KUB 250 fits easily beside incubators in the NICU. The system features an articulating arm for decubitus views and a lightweight DR detector that slides directly into the incubator slot, eliminating the need to move the neonate and reducing stress. Additionally, as a dedicated system for the NICU, the KUB 250 decreases risk of cross-contamination from other areas of the hospital.
For more information: www.kubtec.com


 January 22, 2026
January 22, 2026