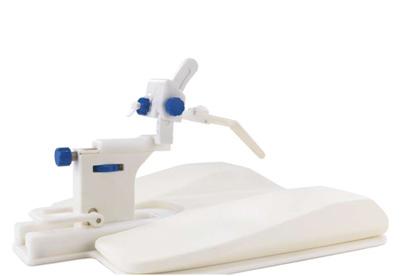
August 12, 2009 - Invivo Corp. launches DynaTRIM, a first-of-its-kind fully MR compatible interventional device for Trans-Rectal Interventional MR (TRIM) biopsy of the prostate gland.
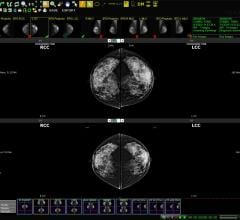
This complete clinical MR solution for prostate combines new DynaCAD for Prostate, an advanced clinical visualization system, and new DynaTRIM, a device for the first MR-guided intervention application. The combined solution offers a truly new imaging and intervention option for many patients with elevated and/or rising PSA levels.
Over the past five years, at the following clinical sites - Fox Chase Cancer Center, Philadelphia, Penn., Radboud University of Nijmegen, The Netherlands, University of Chicago, Ill., and Desert Medical Imaging, Indian Wells, Calif. - clinicians have assisted Invivo in the evaluation of this software with its comprehensive set of advanced prostate image visualization tools for performing real-time image analysis and interventional procedure planning.
“DynaCAD for Prostate provides increased operational efficiency enabling the radiologist to rapidly interpret the 3,000 images that constitute a dynamic contrast enhanced prostate MRI,” said John Feller, Chief Medical Officer at Desert Medical Imaging.
The Urologist faces the dilemma of patients with elevated and/or rising PSA levels and negative TRUS-guided biopsy results. The Invivo complete clinical MR prostate solution is designed to offer an alternative to keeping patients in an uncertain state. DynaTRIM reportedly enables the physician to conduct targeted MRI-guided interventions of the prostate gland reducing the number of cores acquired during biopsy. “Especially for patients that have had several sessions of biopsies with negative results; they only need a maximum of 4 MR-guided biopsies with a detection rate of 59 percent,” according to Dr. Inge von Oort, Onocological Urologist, University of Medical Center Nijmegen, the Netherlands.
For more information: www.invivocorp.com

 July 07, 2021
July 07, 2021