Biomarker blood test accurately confirms remission in non-smoker with HPV-associated oral cancer.
October 30, 2018 — A highly sensitive blood test that detects minute traces of cancer-specific DNA has been shown to accurately determine whether patients with human papilloma virus (HPV)-related oropharyngeal squamous cell carcinoma (OPSCC) are free from cancer following radiation therapy. Findings were presented at the American Society for Radiation Oncology (ASTRO) 2018 annual meeting.
The sophisticated, new liquid biopsy test – which measures fragments of DNA shed by cancers cells in the blood – could save thousands of dollars per patient by reducing the need for costly radiological studies such as PET/CT scans following radiation therapy and greatly increase patients’ peace of mind.
“We’ve developed a highly specific, sensitive liquid biopsy blood test for people with HPV-associated OPSCC,” said Bhisham Chera, M.D., an associate professor of radiation oncology at the University of North Carolina at Chapel Hill and a member of the UNC Lineberger Comprehensive Cancer Center. “This blood test had exceptional performance in monitoring patients for cancer recurrence after radiation therapy. If the circulating tumor HPV DNA is undetectable, there is a high likelihood that the patient is in remission and cancer-free.”
The number of oral cancer cases associated with HPV has been climbing over the past several decades, even as head and neck cancers have generally been declining. Oropharyngeal SCC is the most common HPV-related cancer, and it is five times more common among men than women. This type of cancer affects the back of the throat, including the base of the tongue and tonsils, and it is most prevalent in younger, non-smoking men (median age of diagnosis in the 50s). Radiation therapy has been shown to be a highly effective treatment, but patients must be monitored for recurrence for up to five years to ensure their cancer doesn’t return.
The liquid biopsy test developed by Chera and his colleagues to monitor patients following radiation therapy is a digital polymerase chain reaction assay for HPV DNA that is highly specific, in that it does not cross-detect other types of cellular DNA; precise, in that it can be consistently reproduced; and sensitive, in that it has the ability to detect as few as six molecules of HPV DNA in a blood sample.
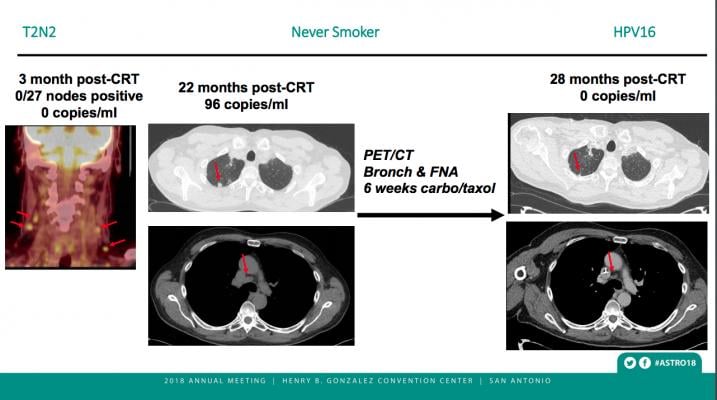
The test was able to predict whether a patient was cancer-free with very high accuracy, indicated by a negative predictive value — the probability that patients with a negative screening test truly don’t have cancer — of 100 percent. That type of accuracy in a liquid biopsy test, said Chera, could potentially reduce the need for costly radiological studies during post-treatment surveillance, reserving them only for patients with detectable ctHPVDNA. The cost for a liquid biopsy test of this type is in the hundreds of dollars, compared to several thousand for radiological studies.
In other types of liquid biopsy tests, explained the researchers, it can be difficult to differentiate between DNA coming from normal cells and that coming from tumor cells, making it tough to get an accurate picture.
“Viruses circulating in the body can be derived either from tumors or from normal infections, and a key challenge for post-treatment care is distinguishing between these two very different sources,” said Gaorav P. Gupta, M.D., Ph.D., an assistant professor of radiation oncology at UNC and also a member of the Lineberger Cancer Center. “Our approach makes it possible to distinguish between tumor-derived and non-tumor sources of HPV.”
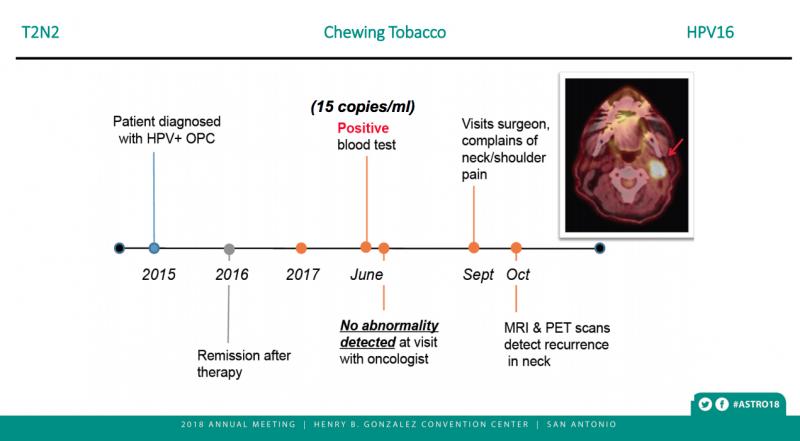
Their multianalyte assay also tests the same piece of DNA twice, allowing for verification, said Dr. Gupta. “As a result, we can now detect minute amounts of cancer in patients from a simple blood draw. In our clinical study, every patient who developed a recurrence scored positively for the test – in some cases many months before their tumors would be clinically detectable. Not a single patient that was negative for our test developed a recurrence.”
The test also uses technology that allows for a more precise count of how many pieces of tumor DNA are in the blood at any given time, said Chera. “You can see the number of fragments growing or declining, and we can make comparisons over time. Older techniques gave you a relative quantification and had limited sensitivity. Our test is providing absolute quantification, so you can actually measure how much tumor-derived HPV DNA is there each time.”
This prospective biomarker trial included 89 patients with HPV-associated OPSCC whose cancer had not spread distantly to other organs. All patients received definitive chemoradiation therapy (CRT), with 78 receiving de-intensified CRT to 60 Gray (Gy) total radiation dose and 11 receiving standard CRT to 70 Gy.
Beginning three months after treatment completion, patients were monitored with a combination of PET/CT scans, CT scans and chest x-rays every six months. Additionally, patients underwent clinical exams every two to four months for two years, and every six months for the following three years. The average follow-up time for patients on the study was 19.8 months (range: 3.7–44.7).
Blood was drawn and tested for ctHPVDNA in all patients during each follow-up visit after treatment. If ctHPVDNA was detected, additional imaging tests were performed.
Following the three-month, post-CRT scans, 70 of the 89 patients in the surveillance cohort had undetectable levels of ctHPVDNA at every follow-up visit. None of these 70 patients showed any sign of cancer recurrence. The remaining 19 patients developed a positive ctHPVDNA test result with a median interval from CRT of 16.7 months (range: 7.8–30.4) and a median value of 75 copies/mL (range 9–28,369). Eight of the patients who developed a positive test result were diagnosed with cancer recurrence (0 local, 1 regional, 7 distant). The other 11 patients showed detectable levels of ctHPVDNA (range: 23–28,369 copies/ml) but no other evidence of cancer recurrence; they are being monitored with repeat blood tests and imaging.
The test could provide patients with much-needed peace of mind, said Dr. Chera. “We are showing in this abstract that the blood test performs very well. It detects cancer before the scan detects cancer. Using this test, I can walk into a patient’s room and say, ‘You are more than likely cancer-free at this point.’”
In the future, ctHPVDNA testing may also potentially be used to screen for cervical cancer or anal cancer, which are also frequently associated with HPV infection, the authors said. They recommend further research to see if it could improve early detection of cancer recurrence and reduce costs by targeting radiographic surveillance to the subset of patients who are at greatest risk of relapse.
Intellectual property related to the test and held by the University of North Carolina at Chapel Hill has been licensed to Naveris, a company in which Chera and Gupta hold equity stakes.
Read more about the Late-breaking Radiation Therapy Clinical Trials at ASTRO 2018.
For more information: www.astro.org


 February 04, 2026
February 04, 2026 









