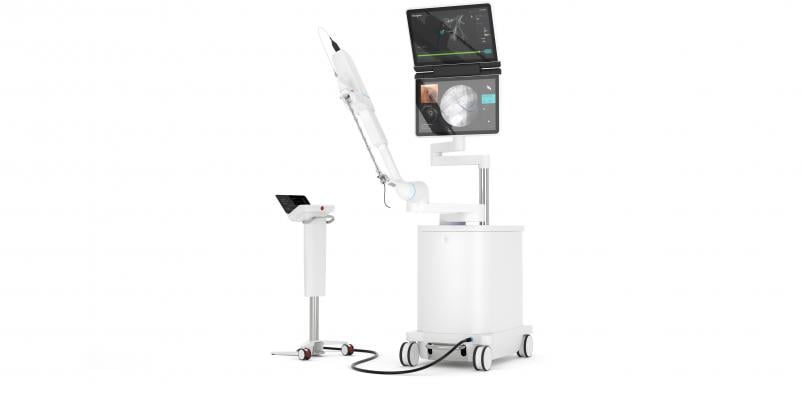
February 20, 2019 — The U.S. Food and Drug Administration (FDA) cleared the Ion endoluminal system from Intuitive Surgical Inc. to enable minimally invasive biopsy in the peripheral lung.
Lung cancer is the world’s leading cause of cancer deaths, according to Intuitive Surgical. Many suspicious lesions found in the lung may be small and difficult to access, which can make obtaining a diagnosis challenging.
The Ion system uses an ultra-thin articulating robotic catheter that can move 180 degrees in all directions. The outer diameter of the catheter is 3.5 mm, which physicians can navigate through small and tortuous airways to reach nodules in any airway segment within the lung. The system’s flexible biopsy needle (called the Flexision Biopsy Needle) can also pass through very tight bends via Ion’s catheter to collect tissue in the peripheral lung. The catheter’s 2mm working channel can also accommodate other biopsy tools, such as biopsy forceps or cytology brushes, if necessary.
Intuitive developed the system’s fiber optic shape sensor technology to provide the physician with the precise location and shape information of the catheter throughout the navigation and biopsy process. The system is designed to easily integrate into existing lung nodule biopsy workflows, as well as existing imaging technology, including fluoroscopy, radial-endobronchial ultrasound and cone-beam computed tomography (CBCT).
Intuitive plans to introduce the Ion system in the U.S. in a measured fashion, with customer shipments beginning in the second quarter of 2019.
For more information: www.intuitive.com


 March 05, 2026
March 05, 2026