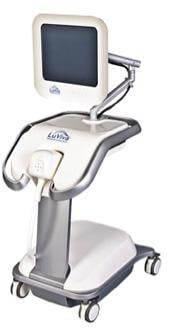
January 29, 2016 — Guided Therapeutics Inc. announced that Nairobi County Health Services Sector has agreed to purchase an additional five LuViva Advanced Cervical Scans for use in the agency’s cervical cancer screening program.
The five LuVivas are expected to ship in Q1 2016. The planned purchase brings the number of LuVivas purchased by Nairobi County to six, with an additional seven units planned for purchase in 2016. When fully implemented, the county will have the capacity to screen more than 12,000 women per month.
“With its immediate results and ease of use, we believe that LuViva is the best option for screening for cervical cancer,” said Nairobi County Chief Officer for Health Robert Ayisi, M.D. “Routine screening, which leads to early detection, combined with other prevention measures, is the best way to reduce the incidence of cervical cancer, which is a major problem in East Africa.”
Cervical cancer ranks number one in cancers of women in Kenya. According to the World Health Organization (WHO) and The International Cancer Organization (ICO) Information Centre, there are 10.32 million women in Kenya at risk of developing cervical cancer. The annual number of women diagnosed with cervical cancer is 2,454, with 1,676 dying every year. It is estimated that there are 15 new cases of cervical cancer diagnosed each week in Nairobi County. These statistics show that an estimated 12.7 women out of every 100,000 are affected.
LuViva is a diagnostic device that scans the cervix with light and uses spectroscopy to measure how light interacts with the cervical tissue. Spectroscopy identifies chemical and structural indicators of precancer that may be below the surface of the cervix or misdiagnosed as benign. This technique is called biophotonics. Unlike Pap, HPV tests or biopsies, LuViva does not require laboratory analysis or a tissue sample, and is designed to provide results immediately, which may result in eliminating costly, painful and unnecessary additional testing.
LuViva is intended for use with women who have undergone initial screening and are called back for follow-up with a colposcopy examination, which in many cases, involves taking a biopsy of the cervix. It has also been used in clinical studies in Turkey and Nigeria as a means to screen women for cervical cancer where the availability of infrastructure necessary for Pap and HPV testing is restricted. The device is used in conjunction with the LuViva Cervical Guide single-use patient interface and calibration disposable.
For more information: www.guidedinc.com


 February 18, 2026
February 18, 2026 









