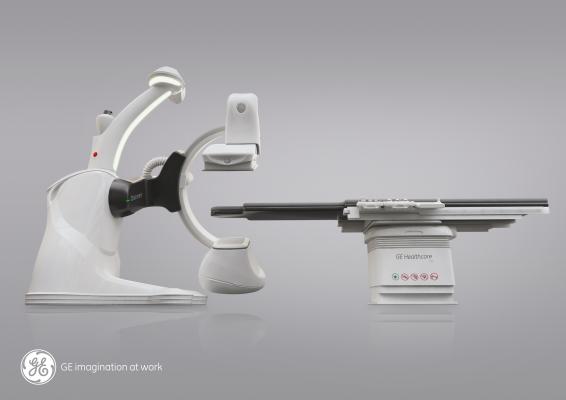
December 13, 2013 — At RSNA 2013, GE Healthcare introduced the Discovery Interventional Guidance System (IGS) 740, a 510(k)-pending mobile X-ray angiography system with a 41 x 41 cm detector. The system is aimed at interventional radiology market.
The rail-free design aims to allow healthcare professionals ample access to the patient while freeing clinical teams from the constraints of fixed ceiling-mounted system rails. Its wide bore C-Arm and dedicated arm-imaging positions are designed to enable ease in imaging the anatomy of interest, and full patient access from left or right. By eliminating the ceiling rails, installation is intended to be simplified for flexibility in designing the room and positioning ceiling-mounted ancillaries (monitors, rad-shields and lights) where healthcare professionals need them.
“Our goal was to pioneer a solution designed to free interventional radiologists from traditional constraints,” said Chantal Le Chat, general manager of GE Healthcare Premium Angiography. “With the enhanced system mobility of the Discovery IGS 740, clinicians will have full freedom to operate, and we believe this will revolutionize the field of interventional imaging.”
GE introduced the first of its IGS series mobile angiography systems at RSNA 2011 and commercialized it in 2012. The IGS series uses a laser guided positioning system to enable it to move anywhere in a room without the need for cables and mounting points. It offers the advantages of high image quality, software and rotational angiography offered by fixed based systems, but the mobility of a mobile C-arm.