January 6, 2012 – A firm developing a noninvasive test for the early detection of lung cancer and other applications today reported that it had achieved full automation of its Cell-CT system - a proprietary imaging platform that generates high-resolution 3-D biosignatures from intact cells. Development of the fully automated system, by the company, VisionGate, Inc., was funded in part by a $2.6 million grant from the National Institutes of Health's (NIH) Biomedical Research, Development, and Growth to Spur the Acceleration of New Technologies program (BRDG-SPAN).
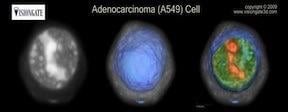
VisionGate's first diagnostic application for the automated Cell-CT system is the LuCED test, a non-invasive test in development for lung cancer screening. LuCED and the Cell-CT platform produce detailed 3-D images of cells in sputum, which the system automatically analyzes to identify key features, or biosignatures, associated with potential malignancy. The analysis yields a score that indicates whether or not cancer cells are present. The Cell-CT system produces clear and comprehensive 3-D images of the cells, enabling extremely accurate classifications. LuCED is initially being developed for use in conjunction with x-ray CT screening, which has been shown to reduce lung cancer deaths in high-risk individuals, but which also has a high rate of false positive results. LuCED is expected to greatly reduce the incidence of false positives, potentially enabling the approach to be used for cost-effective lung cancer screening on a mass scale.
“We believe the automated Cell-CT platform could revolutionize cell-based diagnostics, both by enabling early disease detection and by providing pathologists with a powerful new tool for a wide range of applications,” said Alan Nelson, Ph.D, chairman and CEO of VisionGate. "In the near-term, automation of the Cell-CT platform sets the stage for submission of our first applications to the Food and Drug Administration (FDA) later this year for regulatory review of LuCED."
In a July 2011 presentation at the International Academy for the Study of Lung Cancer's 14th World Conference on Lung Cancer, VisionGate showed how LuCED and the Cell-CT platform could accurately detect cancer cells in sputum samples from individuals at high risk of lung cancer.
"The Cell-CT 3-D imaging system could be a valuable tool for early detection of lung cancer, when the disease is potentially curable," said David Yankelevitz, M.D., professor of radiology and director of the lung biopsy service at the Mount Sinai Medical Center in New York City. "But the large number of individuals at high risk for developing this disease, which kills more than 160,000 Americans annually, mandates that screening be accurate, efficient and cost effective. Automation of this innovative diagnostic tool is an important step towards achieving these performance requirements."
The Cell-CT automated system uses optics and computational technology that have the capability to capture images rapidly and render scanned objects into 3-D digital images. All components of the Cell-CT system have been custom-designed by VisionGate's and the technology is covered by 66 issued patents around the globe.
The BRDG-SPAN program aims to accelerate the transition of research innovations and technologies toward the development of products or services that will improve human health, help advance the mission of NIH and its affiliates, and create significant value and economic stimulus. This program also aims to foster partnerships among a variety of R&D collaborators working toward these aims.
VisionGate will be presenting at the 2012 OneMedForum Finance Conference in San Francisco on Tuesday, January 10, 2012.
For more information: www.visiongate3D.com, http://www.nhlbi.nih.gov/recovery/funding/small-biz-prog.htm


 February 09, 2026
February 09, 2026 









