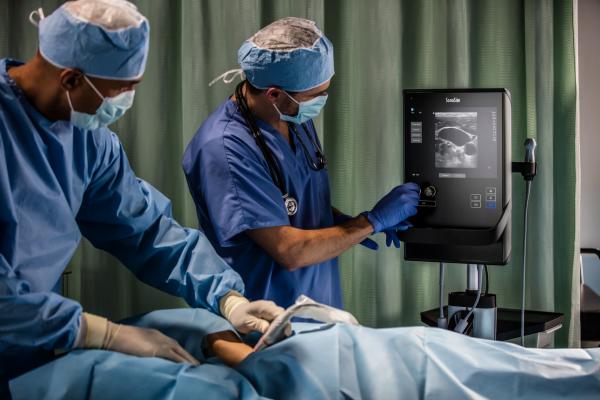
Fujifilm SonoSite Inc., received its CE mark and 510(k) clearance for the new mountable ultrasound system, the SonoSite SII. Developed for regional anesthesia, vascular access and trauma applications, the SII empowers efficiency for clinicians through a simple, yet smart user interface that adapts to the user’s imaging needs. The system is portable and can be used across multiple hospital environments, including a zero-footprint option for space-constrained rooms.
“SonoSite introduced the first mountable ultrasound system in 2007, providing an unparalleled solution for clinicians who valued and needed to accelerate their clinical workflow,” said Brian Leck, Vice President, Global Direct Sales, Fujifilm SonoSite Inc. “The new SII ultrasound system expands on the design goals of our mountable legacy system by offering more functionality, and an even better user experience from start to finish. We listened to clinicians, and delivered a product designed to maximize the efficiency of their ultrasound use. The SII captures the epitome of the SonoSite brand, allowing clinicians to confidently use the system from day one.”
For regional anesthesiologists, enhancing patient throughput is a critical need, especially as they perform an increasing number of ultrasound-guided procedures on a daily basis. The SII features a new touch screen user interface with a clinician-driven menu logic that adaptively adjusts to the use case – “what you need, is what you see.” An embedded dual transducer connector also allows quick switching between transducers with two simple taps of the screen, ensuring that the right transducer is always readily available. To further accelerate end-to-end workflow, the SII comes with a new stand, offering elevated transducer holders and additional storage, all while minimizing footprint.
For trauma patients, the speed and ease of image acquisition is vital, as a few minutes can alter a patient’s care path. The SII features DirectClear technology, a novel, patent-pending process that is available on select transducers. DirectClear elevates transducer performance by increasing penetration and contrast resolution. This transducer innovation contributes to an unsurpassed imaging experience for the clinician.
The SonoSite SII transforms the pace of patient care for proceduralists or those clinicians requiring a quick answer at a critical moment.
For more information: www.sonosite.com.


 February 05, 2026
February 05, 2026 









