October 25, 2013 — The U.S. Food and Drug Administration (FDA) today approved Vizamyl (flutemetamol F 18 injection), a
radiopharmaceutical tracer, for use with positron emission tomography (PET) imaging of the brain in adults being evaluated for Alzheimer's disease (AD) and dementia. The agent was developed for GE Healthcare by Medi-Physics Inc.
Dementia is associated with diminishing brain functions such as memory, judgment, language and complex motor skills. The dementia caused by AD is associated with the accumulation in the brain of an abnormal protein called beta amyloid, and damage or death of brain cells. However, beta amyloid can also be found in the brain of patients with other dementias and in elderly people without neurologic disease.
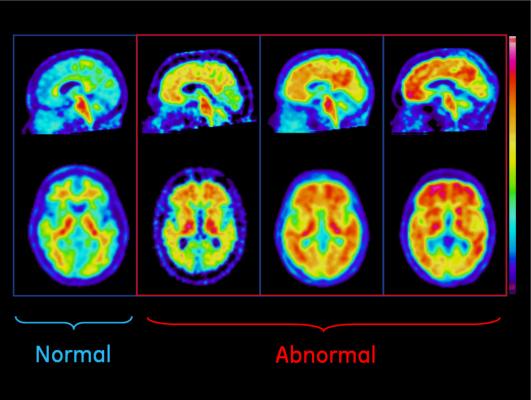
The FDA said Vizamyl works by attaching to beta amyloid and producing a PET image of the brain that is used to evaluate the presence of beta amyloid. A negative Vizamyl scan means that there is little or no beta amyloid accumulation in the brain, and the cause of the dementia is probably not due to AD. A positive scan means that there is probably a moderate or greater amount of amyloid in the brain, but it does not establish a diagnosis of AD or other dementia. Vizamyl does not replace other diagnostic tests used in the evaluation of AD and dementia.
“Many Americans are evaluated every year to determine the cause of diminishing neurologic functions, such as memory and judgment, that raise the possibility of Alzheimer’s disease,” said Shaw Chen, M.D., deputy director of the Office of Drug Evaluation IV in the FDA’s Center for Drug Evaluation and Research. “Imaging drugs like Vizamyl provide physicians with important tools to help evaluate patients for AD and dementia.”
Vizamyl is the second diagnostic drug available for visualizing beta amyloid on a PET scan of the brain. In 2012, the FDA approved
Amyvid (Florbetapir F-18 injection) to help evaluate adults for AD and other causes of cognitive decline.
Vizamyl’s effectiveness was established in two clinical studies comprised of 384 participants with a range of cognitive function. All participants were injected with Vizamyl and were scanned. The images were interpreted by five independent readers masked to all clinical information. A portion of scan results were also confirmed by autopsy.
The study results demonstrate that Vizamyl correctly detects beta amyloid in the brain. The results also confirm that the scans are reproducible and trained readers can accurately interpret the scans. Vizamyl’s safety was established in a total of 761 participants.
Vizamyl is not indicated to predict the development of AD or to check how patients respond to treatment for AD. Vizamyl PET images should be interpreted only by health care professionals who successfully complete training in an image interpretation program. The Vizamyl drug labeling includes information about image interpretation.
The FDA said safety risks associated with Vizamyl include hypersensitivity reactions and the risks associated with image misinterpretation and radiation exposure. Common side effects associated with Vizamyl include flushing, headache, increased blood pressure, nausea and dizziness.