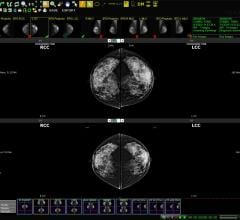
May 7, 2008 – The FDA cleared a new imaging device, ei•Nav/Artemis, that uses 3D/4D imaging to allow urologists to virtually see inside the prostate in real time during biopsy, guiding them with 4D needle navigation during the procedure, mapping biopsy locations and generating an image of 3D biopsy coordinates for future reference.
The system’s 3D/4D imaging allows doctors to select and biopsy a location within the boundary of the prostate with pinpoint accuracy. The biopsy location is then recorded by Artemis’ patented registration technology, which allows doctors to revisit or avoid the exact same area during repeat procedures. Artemis provides doctors with data they can analyze to determine if the prostate gland has changed and manage treatment accordingly.
This imaging and mapping is reportedly a major improvement over existing 2D ultrasound routinely used for prostate cancer biopsies, where doctors blindly biopsy cells and roughly estimate locations during repeat procedures. Without being able to clearly see the prostate in real time, doctors have had no choice but to gather less-than-precise information to determine treatment.
The first Artemis Imaging systems will be in use at the University of Colorado Health Sciences Center and UC San Francisco within weeks and are available for immediate order in the U.S.
For more information: www.eigen.com

 July 07, 2021
July 07, 2021