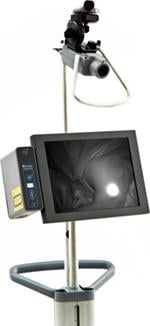
August 30, 2010 - The U.S. Food and Drug Administration (FDA) granted clearance for a catheter guidance system. Designed to aid in the placement of peripherally inserted central catheters (PICC), SonoSite’s LumenVu combines near-infrared technology with a revolutionary fiber optic stylet, which replaces a traditional guide wire, to allow visualization and real-time tracking of a catheter tip as it advances through a vein.
An estimated 2.5 million PICC line placements were performed in the United States in 2008(1), making it one of the most rapidly growing applications performed in the hospital setting. Although a common procedure, PICC placements do pose risks and potential complications. A recent University of Pennsylvania Medical Center study found that only 70 percent of PICC lines are successful on the first attempt(2).
The LumenVu System is designed to mitigate these risks by providing clinicians with the ability to visualize the catheter tip as it travels through the vessel. Health care professionals in hospitals or long-term care facilities will be able to track the progression of a catheter with greater confidence and quickly make navigational adjustments.
The LumenVu System is compatible with standard catheter suppliers and is designed to allow clinicians seamless integration of the product into their current PICC line process. Additionally, the LumenVu System does not rely on a magnetic field and will not interfere with medical equipment or with devices in the patient’s body, such as a pacemaker.
For more information: www.sonosite.com/products/lumenvu
(1) N. Moureau, "Vascular Safety: It’s all about PICCs," Nursing Management 2006: Volume 37, Number 5, 22-27
(2) S.O. Trerotola, S. Thompson, J. Chittams, K.S. Vierregger, "Analysis of Tip Malposition and Correction in Peripherally Inserted Central Catheters Placed at Bedside by a Dedicated Nursing Team," Journal of Vascular and Interventional Radiology 2007; 18: 513-18


 February 05, 2026
February 05, 2026 









