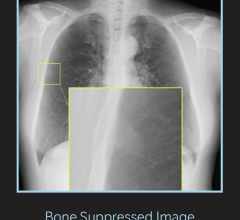
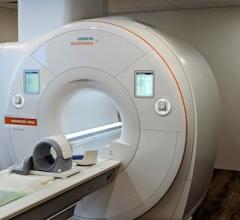
March 20, 2019 — The U.S. Food and Drug Administration (FDA) has cleared the Mobilett Elara Max mobile X-ray system from Siemens Healthineers. The system offers features that enable comprehensive information technology (IT) security as well as secure system integration into the hospital’s IT environment for anytime access to patient data. The system also has an improved design that includes an antimicrobial coating.
The comprehensive security package of the Mobilett Elara Max digital radiography (DR) system helps healthcare facilities handle cyberthreats, while its virtual workstation technology provides access to necessary patient information directly at the bedside. The system’s ergonomic design has an antimicrobial coating and fully integrated cables for an unobstructed front view that allows for smooth, safe maneuverability. Additionally, the system delivers 180-degree lateral arm movement that maximizes positioning options to provide customers with robust imaging power for diagnostic confidence.
As a member of the Max product family, Mobilett Elara Max benefits from the advantages provided by a standardized technology platform, ranging from radiography to fluoroscopy to mobile X-ray imaging. This permits the standardization of workflows across diverse examinations. Through the common use of detectors for different platform systems, customers can achieve considerable savings using systems with the MAX Effect. Identical user interfaces shorten the training period, reduce errors, and increase efficiency.
For more information: www.usa.healthcare.siemens.com

 February 10, 2026
February 10, 2026