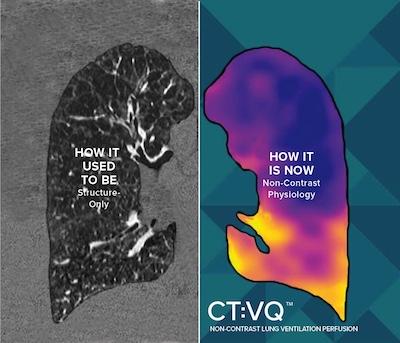
Sept. 4, 2025 — 4DMedical, a global medical technology company, has announced U.S. Food and Drug Administration (FDA) 510(k) clearance for CT:VQ, the world's the company's non-contrast, ventilation–perfusion (V/Q) imaging solution. In parallel, the U.S. Centers for Medicare & Medicaid Services (CMS) has confirmed reimbursement for CT:VQ under Category III CPT codes; this payment is in addition to existing reimbursement for the underlying chest CT.
CT:VQ converts standard, non–contrast chest CTs into quantitative, lobar ventilation (V) and perfusion (Q) maps. Delivered as software–as–a–service (SaaS), it integrates directly with routine radiology workflows (DICOM-based, PACS reporting) and leverages the U.S. installed base of approximately 14,500 CT scanners, bringing functional lung imaging to sites without nuclear medicine capacity.
"CT:VQ gives clinicians all the contrast—and none of the injections," said Andreas Fouras, PhD, founder and CEO of 4DMedical. "With FDA clearance and Medicare payment in place, any hospital with a CT scanner can turn a routine chest CT into a high–resolution ventilation–perfusion study in minutes, without new hardware or workflow complexity. The word 'breakthrough' is overused, but we believe the unprecedented capabilities of CT:VQ qualify for that description."
CT:VQ transforms a routine non-contrast chest CT into a reimbursable V/Q study, eliminating the need for new hardware. Patients skip injections and complete the entire process in a single CT appointment. Radiologists then receive high-resolution, quantitative V/Q maps directly in PACS. This allows pulmonologists to gain actionable information for PE workups, CTEPH assessment, COPD phenotyping, BLVR planning, and ongoing monitoring. Since CT:VQ operates on existing scanners, hospitals and imaging centers, including those without nuclear medicine, the technology can be implemented to immediately expand access to community and rural patients.
More than one million nuclear V/Q scans are performed annually in the U.S. 4DMedical's clinical validation for CT:VQ included quantitative performance testing against SPECT, expert reader studies, and real–world cases across multiple lung conditions. Early U.S. clinical partners have included Stanford University and Brooke Army Medical Center, with the later presenting initial findings at the recent 2025 American Thoracic Society meeting.
Learn more at www.4dmedical.com.


 February 06, 2026
February 06, 2026 









