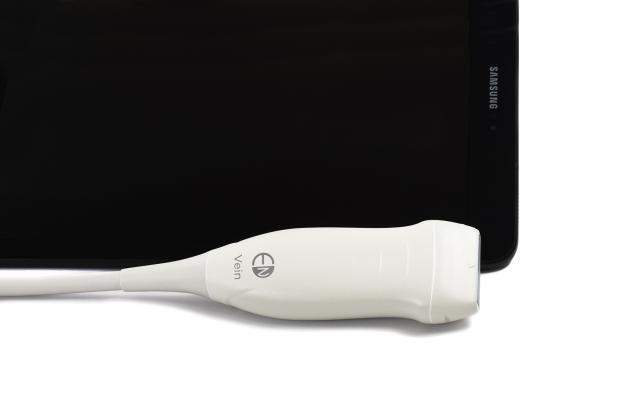
July 12, 2018 — EchoNous has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the EchoNous Vein, an ultrasound-based tool designed specifically for nurses to improve peripheral IV (PIV) catheter placements. Developed for usage across a wide range of patients including both adults and children, EchoNous Vein provides immediate, clear images at depths from 1 to 5 centimeters for quickly visualizing superficial and deeper veins with just two-button controls. EchoNous Vein will integrate with the company’s existing intelligent medical tool, Uscan, to form the EchoNous platform.
One of the most commonly performed minimally-invasive procedures, peripheral IVs can be challenging due to chronic illness, chemotherapy, obesity and drug abuse; first-attempt IV catheter insertion fails in up to 26 percent of adults and 54 percent of children1.
Early feedback from healthcare providers has shown the system’s unique frequency profile and optimized gain and depth presets have the potential to provide significant advancements in evaluating veins of pediatric patients – a traditionally challenging patient population for IV insertion.
“EchoNous Vein’s simple on-screen controls allows clinicians using ultrasound to clearly identify veins in the center of the display, helping to not just locate veins, but to evaluate their health and quality prior to peripheral IV placement,” said Nancy Moureau, RN, Ph.D., CEO of PICC Excellence, Inc. “As clinicians we know that IV insertion selecting a healthy vein for catheter placement can help to reduce the chance of post insertional complications. To have a tool specifically designed to easily select veins and guide catheter placement is extremely valuable as we’re always looking to improve the patient experience.”
In addition to launching the new vascular access tool, the company also announced its plan to converge its Signostics brand and artificial intelligence (AI)-driven Uscan bladder scanner product under the single EchoNous brand.
For more information: www.echonous.com
Reference
1. Helm, Robert E., et al. Accepted but Unacceptable: Peripheral IV Catheter Failure. Journal of Infusion Nursing. 2015 May-Jun;38(3):189-203.


 February 05, 2026
February 05, 2026