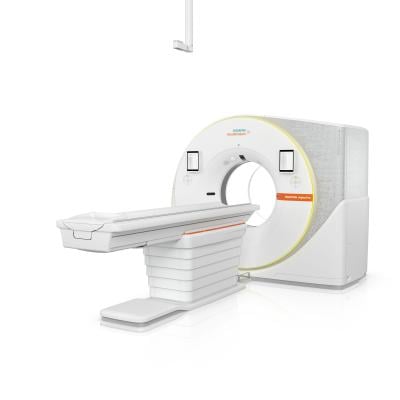
Sept. 4, 2025 — University Hospitals health system in Cleveland recently became the first healthcare institution in the United States to install the Naeotom Alpha.Pro from Siemens Healthineers, the world’s second dual-source scanner with photon-counting computed tomography (PCCT) technology.
PCCT registers each X-ray photon that passes through the patient to acquire more detailed anatomical images with functional information. With this technology, clinicians can evaluate small structures precisely and with fewer artifacts using lower radiation doses than conventional CT. Since Siemens Healthineers introduced the first clinically available PCCT scanner, the dual-source Naeotom Alpha (now the Naeotom Alpha.Peak), in 2021, over 2 million patients worldwide have been scanned with PCCT.
“We are excited to be the first healthcare system in the U.S. to install this impressive new technology to further enhance care for our patients,” said Donna Plecha, MD, chair of the department of radiology, radiologist-in-chief, and Ida and Irwin Haber and Wei-Shen Chin, MD Chair in Radiology at University Hospitals. “The low-dose capabilities of the Naeotom Alpha.Pro, as well as its potential to eliminate patient sedation through fast scanning, will benefit our pediatric patients at UH Rainbow Babies & Children’s Hospital. Additionally, we anticipate using the scanner for some of our patients in UH Cleveland Medical Center’s emergency department, which supports a Level I Trauma Center.”¹
“Siemens Healthineers introduced the Naeotom Alpha.Pro as part of our new class of photon-counting CT scanners to address what we see as our responsibility: to broaden the availability of this groundbreaking technology,” said Matthew Dedman, Head of Computed Tomography at Siemens Healthineers North America. “With University Hospitals becoming the first U.S. health system to install this dual-source PCCT system, we take a major step toward realizing our goal of expanding patient access to the many diagnostic benefits of PCCT.”
The Naeotom Alpha.Pro combines PCCT’s precision with the speed of dual-source technology using AI-powered productivity. It was developed as a lower cost entry point to dual-source PCCT and can achieve scan speeds as fast as 491 mm/sec with the same 66 ms temporal resolution as the Naeotom Alpha.Peak. This high level of speed can benefit pulmonology patients, by reducing the need for breath-holding; pediatric patients, by reducing the need for sedation; and cardiology patients, by reducing the need for beta-blockers to slow their heart rate. Dual-source PCCT scanning supports complex tasks such as automatic analysis and evaluation of highly calcified coronary arteries as well as time-critical stroke and trauma treatments.
Additional information on the Naeotom Alpha class of PCCT scanners can be found at www.siemens-healthineers.com/en-us/computed-tomography/naeotom
¹ The statements by Siemens Healthineers customers described herein are based on results that were achieved in the customer’s unique setting. Because there is no “typical” hospital or laboratory and many variables exist (e.g., hospital size, samples mix, case mix, level of IT and/or automation adoption), there can be no guarantee that other customers will achieve the same results.


 February 04, 2026
February 04, 2026 









