May 5, 2016 — Carestream now offers an oncology reading workflow for positron emission tomography (PET)/computed tomography (CT) studies. The workflow equips clinicians with new native tools for Carestream’s enterprise image management system that deliver both quantitative and qualitative information to help improve and expedite the reading process in tumor detection and tracking.
Carestream’s new CT Perfusion and Subtraction modules contain interactive tools to ease the process of analyzing and comparing three-dimensional (3D), CT perfusion (CTP) and digital subtraction angiography (DSA) images. This equips users to analyze CT brain perfusion scans for tissue perfusion and tissue blood volume, while X-ray angiography (XA) subtraction capabilities provide a DSA workflow for analysis of vessel blockages in areas with bony or dense soft tissue.
“Carestream’s new CT perfusion module that is native to the PACS [picture archiving and communication system] demonstrated better coherence and consistency of perfusion maps and results and was easy to use,” said Daniel Reizine, M.D., radiologist, Department of Neuroradiology, Hospital Lariboisière, Paris, France.
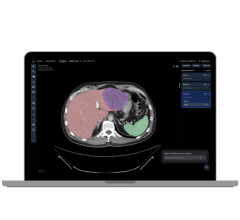
Carestream also developed PET segmentation that allows users to examine tumor standardized uptake values (SUV) traces on the PET image without the need for a physical trace on a CT exam. This capability can play an important role in detection of early stage cancers.
The company’s streamlined oncology reading workflow includes:
- Automatic fusion of PET, single photon emission computed tomography (SPECT), CT and magnetic resonance (MR) images;
- Automatic registration between volumetric datasets that provide efficient comparison of multiple time points;
- Synchronized manipulations, color maps, measurement tools and pre-defined layouts that aid in analysis;
- Reproducible 2-D and volumetric lesion segmentation and automated comparison to observe progression over time;
- Threshold segmentation, spheroid region of interest (ROI), metabolic tumor volume (MTV) and total lesion glycolysis (TLG);
- Bookmarks to catalog lesions and speed future access; and
- Precise follow-up data for enhanced patient care.
An efficient reading workflow is supported by use of Carestream’s native Vue Reporting module. Vue Reporting can present interactive hyperlinks to critical images and automatic inclusion of quantitative analysis in the form of easy-to-understand comparison tables and charts.
Vue Reporting also offers bookmarks and lesion management reporting in accordance with international standards such as response evaluation criteria in solid tumors (RECIST). Reports and images can be shared using Carestream’s Vue Motion universal viewer that supports viewing on U.S. Food and Drug Administration (FDA)-approved mobile devices.
For more information: www.carestream.com


 February 27, 2026
February 27, 2026