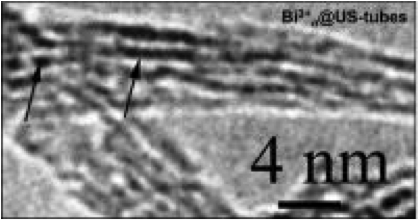
An electron microscope image shows bismuth ions (dark lines) sitting inside carbon nanotubes.
September 5, 2013 — Scientists at Rice University have trapped bismuth in a nanotube cage to tag stem cells for X-ray tracking. Bismuth is probably best known as the active element in a popular stomach-settling elixir and is also used in cosmetics and medical applications. Rice chemist Lon Wilson and his colleagues are inserting bismuth compounds into single-walled carbon nanotubes to make a more effective contrast agent for computed tomography (CT) scanners. Details of the work by Wilson's Rice team and collaborators at the University of Houston, St. Luke's Episcopal Hospital and the Texas Heart Institute appear in the Journal of Materials Chemistry B.
This is not the first time bismuth has been tested for CT scans, and Wilson's lab has been experimenting for years with nanotube-based contrast agents for magnetic resonance imaging (MRI) scanners. But this is the first time anyone has combined bismuth with nanotubes to image individual cells, he said.
"At some point, we realized no one has ever tracked stem cells, or any other cells that we can find, by CT," Wilson said. "CT is much faster, cheaper and more convenient, and the instrumentation is much more widespread [than MRI]. So we thought if we put bismuth inside the nanotubes and the nanotubes inside stem cells, we might be able to track them in vivo in real time."
Experiments to date confirm their theory. In tests using pig bone marrow-derived mesenchymal stem cells, Wilson and lead author Eladio Rivera, a former postdoctoral researcher at Rice, found that bismuth-filled nanotubes, which they call Bi@US-tubes, produce CT images far brighter than those from common iodine-based contrast agents.
"Bismuth has been thought of before as a CT contrast agent, but putting it in nanotube capsules allows us to get them inside cells in high concentrations," Wilson said. "That lets us take an X-ray image of the cell."
The capsules are made from a chemical process that cuts and purifies the nanotubes. When the tubes and bismuth chloride are mixed in a solution, they combine over time to form Bi@US-tubes. The nanotube capsules are between 20 and 80 nanometers long and about 1.4 nanometers in diameter.
"They're small enough to diffuse into the cell, where they then aggregate into a clump about 300 nanometers in diameter," said Wilson. "We think the surfactant used to suspend them in biological media is stripped off when they pass through the cell membrane. The nanotubes are lipophilic, so when they find each other in the cell they stick together."
Wilson said his team's studies showed stem cells readily absorb Bi@US-tubes without affecting their function. "The cells adjust over time to the incorporation of these chunks of carbon and then they go about their business," he said.
Bi@US-tubes have clear advantages over commonly used iodine-based contrast agents, Wilson said. "Bismuth is a heavy element, down near the bottom of the periodic table, and more effective at diffracting X-rays than almost anything else you could use," he said. Once the bismuth is encapsulated in the nanotubes, the agent can produce high contrast in very small concentrations. The nanotube surfaces can be modified to improve biocompatibility and their ability to target certain types of cells. They can also be modified for use with MRI, positron emission tomography (PET) and electron paramagnetic resonance imaging systems.
The Rice lab is currently working to double the amount of bismuth in each nanotube. "Bismuth ions appear to get into the nanotubes by capillary action, and we think we can improve on the process to at least double the contrast, maybe more," Wilson said. "Then we would like to combine both bismuth and gadolinium into one nanotube to produce a bimodal contrast agent that can be tracked with both MRI and CT scanners."
For more information: www.rice.edu


 March 09, 2026
March 09, 2026 









