Contrast media injectors are used to inject contrast media or contrast agents to enhance the blood and perfusion in ...
Contrast Media
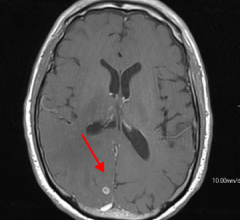
Contrast media, also called contrast agents, are used to enhance the blood and perfusion in tissues. This includes iodine based contrast for on computed tomography (CT), gadolinium based agents for MRI and lipid bubble contrast agents used in ultrasound.
December 11, 2023 — Bracco Imaging S.p.A., an innovative world leader delivering end-to-end products and solutions ...
November 29, 2023 — Bayer continues to advance its comprehensive Radiology portfolio with progress in the development ...
With the use of contrast agents and radiotracers on the rise, GE Healthcare has seen increases in demand across their ...
November 13, 2023 — Bracco Diagnostics Inc., the U.S. subsidiary of Bracco Imaging S.p.A., a pioneer in diagnostic ...
November 3, 2023 — Guerbet, a global leader in medical imaging with more than 30 years of experience in MRI, announced ...
October 17, 2023 — Halo Precision Diagnostics, a leader in early disease detection using precision diagnostics, today ...
Technology advancements are changing consumers’ expectations, driving industries to adapt and evolve, and the healthcare ...
September 21, 2023 — Imaging Biometrics, LLC announced the results of a study that validates IB Neuro’s processing of ...
September 15, 2023 — Contrast enhanced ultrasound (CEUS) is underutilized in the United States, and reduced access to ...
August 17, 2023 — University of Missouri School of Medicine neurologist Adnan Qureshi, MD recently led a study that ...
Steinberg Diagnostic Medical Imaging (SDMI) was founded 30 years ago and has grown to be one of the largest outpatient ...
August 8, 2023 — Fresenius Kabi announced it has launched Gadobutrol Injection, a generic substitute for the contrast ...
July 28, 2023 — Blue Earth Diagnostics, a Bracco company and recognized leader in the development and commercialization ...
July 27, 2023 — Scientists have found a new use for copper in magnetic resonance imaging (MRI) contrast agent design ...
Transitioning to a new device vendor can provide improvements to outdated technology and workflow. It also requires a ...
July 25, 2023 — Bracco Imaging has issued a statement on new results published in the journal Radiology which found that ...
July 7, 2023 — Bayer, a global leader in radiology, has initiated the Phase III clinical development program called ...
July 3, 2023 — According to an accepted manuscript published in ARRS’ own American Journal of Roentgenology (AJR) ...
June 26, 2023 — Bayer announced that Ultravist (iopromide)-300, -370, its iodine-based contrast agent, has been approved ...
June 21, 2023 — Fresenius Kabi, a leading provider of injectable medicines, announced today it has launched the ...
June 20, 2023 — Ascelia Pharma presented data from its Orviglance Hepatic Impairment study during the European Society ...
May 16, 2023 — Bracco Imaging, an innovative world leader delivering end-to-end products and solutions through a ...
May 15, 2023 — Guerbet, a global leader in medical imaging with more than 30 years of experience in MRI, announced that ...

 January 17, 2024
January 17, 2024