There have only been incremental improvements in intravascular ultrasound (IVUS) over the past decade. However, three new technologies introduced in 2010, and a few more expected to enter trials or be commercialized in 2011, are pushing new frontiers. Characterizing Plaque
During a time when the U.S. healthcare reform is on the minds of many within the medical community, physicians and hospital administrators are continuously looking for ways to cut spending. One avenue that physicians and hospitals are exploring is to limit the use of expensive medical devices, such as drug-eluting stents (DES).
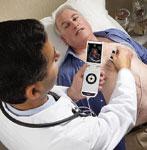
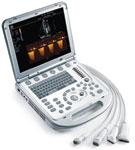
In the same fashion that the echocardiogram helped transform the cardiac exam, recent developments in the field of portable ultrasound are providing cardiologists with a new way to visualize the heart at the initial patient screening.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
Over the past few years, there have been three key areas of advancement in cardiovascular ultrasound technology – miniaturization, 3-D/4-D imaging and quantification measurements.
December 10, 2010 – The 5th annual list of the Top 10 Health Technology Hazards for 2011 is now available. The list, from the ECRI Institute, features the top health technology hazards that warrant critical attention by hospitals and other healthcare organizations.
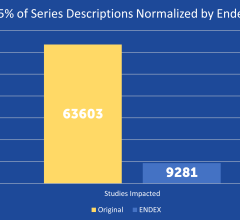
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
December 10, 2010 – The first commercial production of generators using molybdenum-99 (Mo-99) produced with low-enriched uranium (LEU) targets in the United States is underway. The Lantheus TechneLite (Technetium Tc99m) generator received the first commercial scale batch from NTP Radioisotopes, a subsidiary of the Nuclear Energy Corporate of South Africa (NESCA)
While most women understand the importance of health screenings, an estimated 72 million have missed or postponed a ...
Over the past several years, the focus on new technology at the annual Radiological Society of North America (RSNA) scientific meeting in Chicago has moved away from new devices to new software. RSNA 2010, held Nov. 28 through Dec. 2, emphasized this trend.
December 7, 2010 – Four distributors for a PACS and RIS system have been added in Latin America. Novarad has added Electrónica Medica (Panama), Medizintechnik (Chile), Represander (Colombia) and TAG (Guatemala) to represent its NovaPACS and NovaRIS systems in the region and support its sales team. Both systems are available in Spanish and as a Software as a Service (SaaS) offering.
December 7, 2010 – Positron emission tomography/computed tomography (PET/CT) could be a useful tool for predicting local recurrence in lung cancer patients treated with radiofrequency ablation (RFA). Supporting data from a study was published in the December issue of The Journal of Nuclear Medicine.
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
December 6, 2010 – A works in progress data center viewer is available on the iPad. The IMPAX Data Center Viewer, by Agfa, is powered by Xero technology and is designed to enable portable point-of-care to patient images. Clinicians can view reference images, including large multi-slice CT studies, as part of the electronic health record (EHR).
December 6, 2010 – A new, alcohol free disinfectant wipe is safe for use on ultrasound transducers and probes, mammography compressor plates and other non-surgical surfaces. The Protex Disinfectant Wipe, by Parker Laboratories, eliminates bacteria, viruses and fungi with just one wipe.
December 6, 2010 – A panel of medical imaging experts discussed medical imaging appropriateness, ionizing radiation and efforts to curb overutilization, decrease radiation dose and educate patients.
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
December 3, 2010 - A new study reports that the use of computed tomography (CT) in the nation’s emergency departments is growing exponentially. If the growth trend continues, by 2011, nearly 20 percent of all emergency department (ED) visits may involve a CT exam.
December 3, 2010 – A new patient safety E-Lert from the ECRI Institute Patient Safety Organization (PSO) highlights radiation therapy to an unintended site. The safety information comes after several reports submitted by participating heathcare providers.
Acceletronics offers service, spare parts and used/refurbished equipment for the radiation therapy market. At ASTRO 2010, the company also launched its TheraView Classic (CL) electronic portal imaging system.
December 2, 2010 – A new general emergency contacting module has been added to a critical results test management tool. UrgentWorks, by Brit Systems, is now part of the UrgentCall tool and allows users to type general messages for delivery as per their emergency contacting rules.
December 2, 2010 – A cloud-based vendor-neutral storage platform brings together images and metadata from multiple PACS and supports the creation of a master patient index (MPI). The Medweb Cloud can be used for any specialty-based telemedicine and information-management application, including teleradiology, PACS and RIS.
December 2, 2010 – Analyzing historical images in radiology databases can save millions in osteoporosis costs, according to data presented at the 2010 RSNA meeting. Researchers at the Karolinska Institute in Sweden used Sectra’s Digital X-ray Radiogrammetry (DXR) method to identify patients who subsequently suffered a hip fracture.
December 1, 2010 – One of the world's first whole-body MR/PET systems was introduced at the RSNA 2010 meeting. The Biograph mMR, by Siemens, combines an magnetic resonance (MR) imaging scanner with an integrated positron emission tomography (PET) detection system to simultaneously capture both MR and PET data.
December 1, 2010 – A secure advanced visualization application lets physicians access and view critical patient information from anywhere via a Web browser or mobile device. ResolutionMD 2.5, by Calgary Scientific, utilizes the company’s PureWeb technology to ensure that patient information is never duplicated or retained on the device.


 December 13, 2010
December 13, 2010