July 23, 2013 — AFC Industries Inc. announced its Whitestone imaging workstation, now available for the first time in neutral gray. The workstation addresses the need for an integrated workstation with proper adjustable lighting, controlled climate and enhanced acoustics in one ergonomically-friendly unit.
North Carolina joins 11 other states to become the 12th state to standardize dense breast tissue reporting to women, according to a release just issued by Are You Dense Inc./Are You Dense Advocacy, Inc.Addy Jeffrey, Greensboro, North Carolina resident, brought the issue of dense breast tissue to the legislature after her advanced stage cancer diagnosis within 8 months of a normal mammogram. The ACT contained in Chapter 130A of the General Statutes becomes law effective January 1, 2014.
IMRIS has announced U.S. Food and Drug Administration (FDA) 510K clearance to market VISIUS iCT, the first and only ceiling-mounted intraoperative computed tomography (iCT) on the market.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Radiation exposure from multidetector computed tomography (CT) has become a pressing public health concern in both lay and medical publications. Implementation of iterative reconstruction offers the ability to minimize radiation exposure while preserving and, in some cases, improving image quality. However, in order to evaluate iterative reconstruction software, one must first understand the basics of how it works.
Just when positron emission tomography (PET) appears to be eclipsing single photon emission computed tomography (SPECT) for cardiac imaging, new advances make SPECT more attractive. Both modalities also have suffered setbacks with radiopharmaceutical supply problems in recent years and both modalities have their pros and cons. Looking toward the future, the question of which modality will dominate remains unanswered. PET shows major promise with exciting new tracers, while new SPECT scanner technology introduced at the Society of of Nuclear Medicine and Molecular Imaging (SNMMI) 2013 meeting may herald a rebirth for SPECT with previously unseen image quality enhancements.
Healthcare reform requiring wider access and enterprise sharing of patient images and records are making Web-based cardiology picture archiving and communication systems (PACS) a more attractive solution over traditional thick-client, server-based systems. In just the past few years there has been a departure from thick-client cardiology and radiology PACS to Web-based platforms. There are several reasons for this, including better interoperability, anywhere-anytime access, remote access to data and images, and reduction of IT burdens. Web-based systems also enable easier delivery of many healthcare reform Stage 2 meaningful use (MU) requirements.
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
GE Healthcare, hailed a new era for breast imaging today as it announced CE marking for SenoClaire, GE’s new generation of breast tomosynthesis solution designed with a three-dimensional imaging technology. Powered by ASiRDBT, SenoClaire technology uses a low-dose short X-ray sweep around the positioned breast with nine exposures acquired with a “step-and-shoot” method, removing the potential motion from images.
Toshiba America Medical Systems Inc. has partnered with Unfors RaySafe Inc. to offer a new radiation dose monitoring and management tool for Infinix-i cardiovascular X-ray systems. The Unfors RaySafe i2 displays real-time dose exposure information, helping to make exams safer for all clinical staff.
July 22, 2013 — Elekta has received CE marking for its Clarity 4-D monitoring system, permitting European clinics to implement this new way of reducing the uncertainty caused by prostate motion during radiation treatment.
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
Vizzia Technologies announced that its clinical workflow optimization solution was recently installed at Florida Cancer Specialists & Research Institute's (FCS) Tampa Cancer Center location. The newly constructed 35,990 square foot flagship facility offers a "one-stop" approach to patient care that includes medical oncology, advanced radiation oncology technology and radiological services for identifying and diagnosing tumors. The Vizzia solution, which leverages CenTrak's Clinical-Grade RTLS, is designed to transform the outpatient experience and help in optimizing patient flow processes. Shortening patient wait times and ensuring patient safety and satisfaction, as well as increasing the cancer center's patient treatment capacity are among the benefits of the new solution.
July 19, 2013 — Practice Fusion, the largest physician-patient platform in the United States, announced the launch of its Medical Imaging API to digitally connect imaging centers to physicians.
Gamma Medica Inc. has closed a $16 million Series A financing round from healthcare investment firm Psilos Group Managers, LLC. This investment will enable Gamma Medica to expand commercialization of the LumaGEM Molecular Breast Imaging (MBI) system and its companion product the LumaGUIDE MBI-guided biopsy module.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
Viztek announced that MedSpring Urgent Care has recently completed installation of the ViZion Straight Arm DR digital X-ray system at its 14th center, which is located in Chicago. Setting the bar for patient-centered care and providing quality care in a walk-in setting, MedSpring now uses the ViZion Straight Arm DR in all of its facilities, including six in the Austin area, five in the Houston area and three in the Chicago area.
July 18, 2013 — Two reports from AmericanEHR Partners, based on a survey of nearly 1,400 physicians, suggest that tablets are of greater use for clinical purposes than smart phones.
July 18, 2013 — In a study of more than 2,000 adults, researchers found that two MRI (magnetic resonance imaging) measurements of the abdominal aorta — the amount of plaque in the vessel and the thickness of its wall — are associated with future cardiovascular events, such as a heart attack or stroke. Results of the study are published online in the journal Radiology.
Hologic Inc. recently expanded its array of interventional and imaging solutions for breast health with the launch of the world's first 3-D breast biopsy option. This technology was developed for the company's Affirm upright breast biopsy guidance system, which is used in conjunction with the Selenia Dimensions 2-D and 3-D mammography systems.
Elekta and Royal Philips announced that the Froedtert and The Medical College of Wisconsin Clinical Cancer Center (Milwaukee, Wis.) will join a consortium that is investigating the development of a magnetic resonance imaging (MRI) guided radiation therapy system.
July 16, 2013 — The U.S. radiation therapy market appears to be rebounding after a major decline in 2009, with radiation oncology centers budgeting more money for capital equipment purchases this year compared to four years ago, according to a new study published by IMV Medical Information Division.
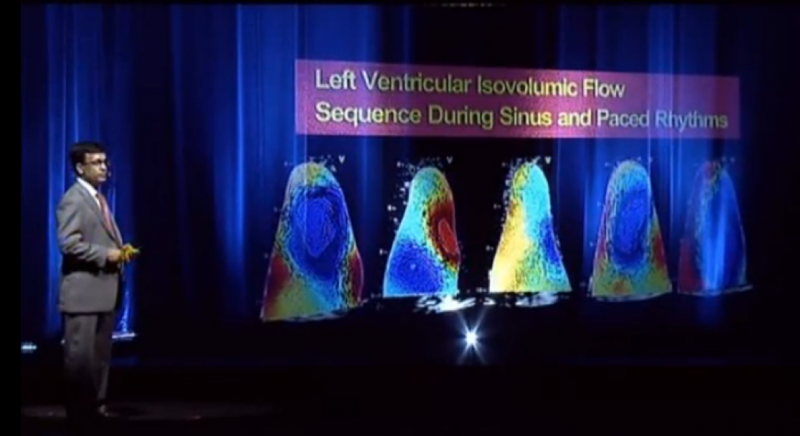
The first-ever presentation at a medical conference using hologram technology instead of traditional video projection screen took place July 1 at the American Society of Echocardiography’s (ASE) 2013 scientific sessions. The innovative presentation was used to drive home the advances in technology that are going to completely reshape cardiac ultrasound and medical imaging over the next few years.
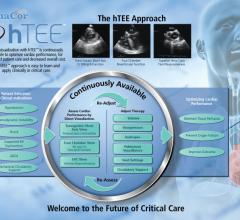
ImaCor Inc. announced the implementation of the 24/7 hTEE Clinical Support Program.


 July 23, 2013
July 23, 2013