TeraRecon announced the availability of a first-of-kind upgrade program called Any/\One, at RSNA 2014.
Helping connect healthcare experts and increasing the usability of the wealth of medical imaging data is the goal of a cloud-based network solution from Siemens Healthcare.
Cianna Medical Inc. received U.S. Food and Drug Administration (FDA) clearance for the SAVI Scout surgical guidance system. The technology uses real-time audible and visual indicators to give surgeons a precise way to target tissue during lumpectomy and excisional biopsy procedures.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Data from a Bayer study in children less than 2 years of age (infants) was presented at the 2014 Radiological Society of North America (RSNA) scientific assembly and annual meeting. The primary endpoint of the study was the evaluation of the pharmacokinetics (PK) of Gadavist (gadobutrol) injection in plasma at the standard dose of 0.1 mmol/kg body weight. Safety was a secondary endpoint and the study also included a qualitative assessment of efficacy.
A new study on the use of hypofractionated whole-breast irradiation (HF-WBI) for early-stage breast cancer patients found HF-WBI increased 17.4 percent from 2004 to 2011. Patients are also more likely to receive HF-WBI compared to conventionally fractionated whole-breast irradiation (CF-WBI) when they are treated at an academic center or live ?50 miles away from a cancer center. The study was published in the Dec. 1, 2014, issue of the International Journal of Radiation Oncology • Biology • Physics (Red Journal), the official scientific journal of the American Society for Radiation Oncology (ASTRO).
iCAD, Inc. announced that more than 500 patients have been treated in its clinical trial of intraoperative radiation therapy (IORT) using the Xoft Axxent Electronic Brachytherapy (eBx) System.
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
A recently published study from Toronto, Canada found that patients who utilized 3-D SPECT were able to get a clearer diagnosis of ADHD, which is the most common psychiatric disorder in children and adults.
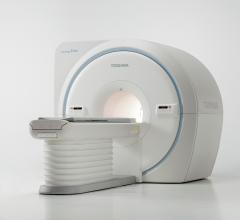
Community hospitals can now provide their patients with a reliable and high-functioning MR system, the Vantage Elan 1.5T from Toshiba America Medical Systems, Inc.
A new lung screening solution from Philips offers healthcare providers a faster and more definitive pathway to lung cancer detection and treatment. Making its debut at the 2014 Radiological Society of North America (RSNA) annual meeting in Chicago, the solution is comprised of products and services that enable healthcare providers to implement and manage a comprehensive computed tomography (CT) lung screening program which tracks and guides patients across the health continuum.
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
Siemens Healthcare introduced the Mobilett Mira Max digital mobile X-ray system at the 100th Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA), Nov. 30-Dec. 5 in Chicago.
Philips launched the Ingenia 1.5T S, a new magnetic resonance imaging (MRI) system designed for "First Time Right" imaging and for faster workflow, while enhancing the patient's experience during MRI examinations.
The new version of the Siemens Somatom Definition Edge CT system, allows dual energy images to be acquired using a single-source system in clinical routine.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
Viztek announced commercial availability of the ViZion + Wireless DR panel, which was shown for the first time at RSNA 2014.
Giving customers the tools to lower dose and ensure safer CT imaging, Toshiba is offering enhancements across its Aquilion One CT product line that improve patient experience and satisfaction.
GE Healthcare’s Signa Pioneer, a new 510(k) pending 3T magnetic resonance imaging (MRI) system, offers a unique feature where it can create six imaging protocols from a single scan. The vendor said this will greatly reduce the amount of time required to image patients and enable an additional patient per hour to be scanned.
As the first integration between Interventional Radiology (IR) and CT technology, Toshiba’s Infinix 4-D CT provides clinicians with faster, safer and more accurate interventions.
ITN discusses the recent trends and advances in breast imaging at RSNA 2014 with Laurie Fajardo, M.D., MBA chief of ...
Researchers from North Carolina State University have developed a technique that allows ultrasound to penetrate bone or metal, using customized structures that offset the distortion usually caused by these so-called "aberrating layers."
NEC Display Solutions of America announced two additions to its MultiSync MD Series portfolio of flat panel displays.
Siemens Healthcare presented two new clinical applications for angiography at the 2014 Radiological Society of North America (RSNA) annual meeting. The syngo Dyna4D software enables time-resolved 3-D imaging in angiography, permitting 3-D visualization of blood vessel volume as well as blood flow. The syngo DynaCT SMART algorithm allows the clinician to remove metal artifacts from patient medical images, potentially enabling the detection of complications such as hemorrhaging that occur in the vicinity of metallic objects contained within the patient’s body.

 December 17, 2014
December 17, 2014