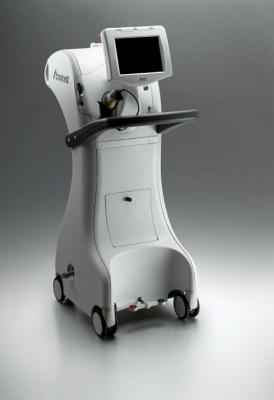
Image courtesy of iCAD
December 16, 2014 — iCAD, Inc. announced that more than 500 patients have been treated in its clinical trial of intraoperative radiation therapy (IORT) using the Xoft Axxent Electronic Brachytherapy (eBx) System. The study, "A Safety and Efficacy Study of Intraoperative Radiation Therapy (IORT) Using the Xoft Axxent eBx System at the Time of Breast Conservation Surgery for Early-Stage Breast Cancer (the ExBRT study)," compares the Xoft System to traditional external beam radiation therapy (EBRT). Patients in the study were treated with a targeted, single-fraction dose of radiation using the Xoft System at the time of lumpectomy.
With the Xoft System, patients are treated with one dose directly to the tumor bed at the time of lumpectomy, thereby reducing the risk to nearby healthy tissue and organs such as the heart, lung and ribs. Using the Xoft System also improves patient quality of life by reducing the number of treatments compared to EBRT, which usually requires 30-35 daily treatments over a period of 5-7 weeks. To date, more than 10,000 patients have been treated globally, across all clinical applications, with the Xoft System.
Researchers plan to enroll up to 1,000 patients across the United States and Europe. Currently, the study includes 23 active centers. Study subjects will be followed for 10 years after treatment to evaluate the long-term safety and efficacy of breast IORT with the Xoft System. The study will also assess cosmetic outcomes and quality of life for those treated with Xoft IORT.
For more information: www.icadmed.com; www.xoftinc.com


 January 30, 2026
January 30, 2026 









