To provide the best possible patient experience with superb image quality, Indiana University Health Goshen Hospital installed the Vantage Titan 1.5T magnetic resonance (MR) system from Toshiba America Medical Systems Inc. Goshen is a part of Indiana’s largest integrated delivery network (IDN) and is utilizing the system in the hospital to support its heart and vascular center to improve patient outcomes with high-quality imaging and a comprehensive suite of patient-focused technologies.
Infinitt North America will showcase a wide variety of radiology solutions at the 2015 Radiological Society of North America (RSNA) annual meeting, Nov. 29-Dec. 4 in Chicago.
Ergonomics plays an important role in the comfort and workflow for sonographers. Ninety percent of sonographers surveyed ...
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Lexmark announced that North Carolina-based UNC Health Care System (UNC HCS) will deploy the Lexmark Vendor Neutral Archive (VNA), NilRead Enterprise Viewer and PACS Scan Mobile solutions at all of its locations. The trio of solutions will help meet diverse image and content management needs, including clinician workflow requirements and integration with the organization’s core patient electronic medical record (EMR) system. UNC HCS produces approximately 1.3 million DICOM studies annually.
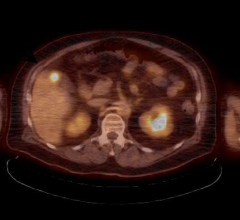
Mirada Medical Ltd, a medical imaging software company, and BTG plc, a global specialist healthcare company, announced a collaboration to develop dosimetry software solutions to optimize radioembolization therapy with TheraSphere.
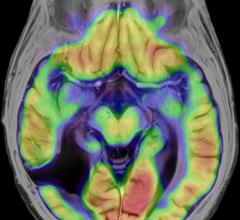
Brainlab showcased cranial Elements for stereotactic radiosurgery (SRS) at the 57th annual meeting of the American Society for Therapeutic Radiology and Oncology (ASTRO). Dovetailing with the society’s 2015 theme, “Technology Meets Patient Care,” Elements provide indication-specific and intelligent workflows for stereotactic radiosurgery that automate the procedure so caregivers can spend more time with patients and less time on technology.
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
Mevion Medical Systems announced that Ackerman Cancer Center (ACC) in Jacksonville, Florida, will install a second Mevion S250 Series proton therapy system. ACC began treating cancer patients with its first S250 system in April, and is already treating enough patients to warrant the installation of a second system. ACC achieved the fastest per-room ramp-up in the history of proton therapy, reaching an annualized rate of 350 patients per year, more than 25 percent better than the average U.S. proton therapy center.
Critical advancements in modern technology will play an integral role in progressing the Future of Healthcare, according ...
The American Society for Radiation Oncology (ASTRO) has awarded the 2015 Annual Meeting Nurses Abstract Award to Bridgett Harr, CPN, Cleveland Clinic, Cleveland, for the abstract “Advanced Practice Nurse Follow-up Clinic Reduces Emergency Room Visits and Admissions in High-Risk Patients after Chemoradiation Therapy for Head and Neck Cancer.”
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
Mach7 Technologies has been named the Asia Pacific Medical Imaging Informatics Company of the Year by global consulting firm Frost & Sullivan. Each year Frost & Sullivan presents this award to the company that demonstrates outstanding achievement and superior performance in the field of medical imaging informatics.
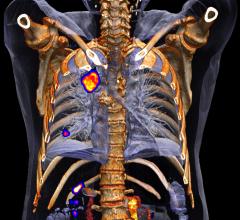
October 28, 2015 — An analysis of an international, cooperative-led trial of patients with locally advanced non-small ...
Augmenix Inc. showcased the SpaceOAR System at the 57th annual American Society of Therapeutic Radiology and Oncology meeting, highlighting robust commercial uptake in the United States. An absorbable hydrogel, SpaceOAR technology is designed to create space and protect the rectum in men undergoing prostate cancer radiotherapy. The device received clearance from the U.S. Food and Drug Administration (FDA) in April 2015 and since then, more than 40 cancer treatment centers in 19 states have adopted it.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
October 28, 2015 — Konica Minolta’s Sonimage HS1 Version 2.0 ultrasound system delivers best-in-class imaging from the ...
The AeroDR LT is a smart wireless digital radiography (DR) solution that can be used in most rooms and portable X-ray systems. The flat panel detector delivers added economic value by extending the life and value of existing radiology rooms and solutions. AeroDR LT is easy to carry and easy to position with its light weight (5.5 lbs.) and grip strips right on the panel—helping increase both patient satisfaction and technologist productivity.
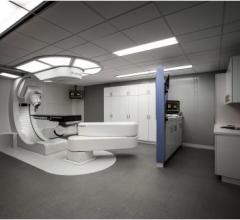
IBA announced that longtime customer and research partner Penn Medicine has upgraded the equipment of its Roberts Proton Therapy Center with the latest innovations in proton therapy treatment. This upgrade to the newest technologies will allow Penn Medicine’s staff to continue to offer top-level proton radiation therapy services to an increasing number of patients and to further advance the clinical application of proton therapy.
Mach7 Technologies and 3D Medical Limited announced yesterday they have signed a definitive agreement to merge together. The merger will provide access via public capital markets for Mach7 while preserving corporate self-determination with regard to mission and product roadmap. Upon approval and completion of the agreement, the merged entity will be publicly listed on the Australian stock exchange (ASX) and will trade as "Mach7 Technologies Ltd."
Carestream Health earned the top rating in MD Buyline’s Market Intelligence Briefing (Q3 2015) for both portable and room-based digital X-ray systems for the third time this year. The company’s DRX-Revolution mobile X-ray system and its DRX-Evolution and DRX-Ascend systems scored high marks from healthcare providers for performance, reliability, installation and service quality.
Healthcare technology company MphRx will debut a new platform at the Radiological Society of North America (RSNA) 2015 annual meeting that enables transmission of detailed patient forms, images and records from any computer for review by providers and automated ingestion into an EMR, PACS or RIS.
University of Missouri researchers have developed a new scoring system for a common lung cancer diagnostic test that may help physicians better understand the risk for malignancy when evaluating patients.
New data from clinical trials conducted at Penn Medicine’s Robert Proton Therapy Center demonstrate the technology's potential advantages over conventional radiation, including less side effects and survival in some cases, for several harder-to-treat tumors. This includes pancreatic, late-stage non-small cell lung and chordoma and chondrosarcoma, two rare cancers found in bone or soft tissue.

 October 30, 2015
October 30, 2015