Image courtesy of Augmenix Inc.
October 28, 2015 — Augmenix Inc. showcased the SpaceOAR System at the 57th annual American Society of Therapeutic Radiology and Oncology (ASTRO) meeting, highlighting robust commercial uptake in the United States. An absorbable hydrogel, SpaceOAR technology is designed to create space and protect the rectum in men undergoing prostate cancer radiotherapy. The device received clearance from the U.S. Food and Drug Administration (FDA) in April 2015 and since then, more than 40 cancer treatment centers in 19 states have adopted it.
“For decades, attempts to limit rectum radiation injury in men receiving prostate cancer radiotherapy included advances in planning and delivery, along with improvements in prostate localization and enhanced image guidance. As we observe increasing interest worldwide in high-dose, ultra-hypofractionated treatment delivery regimens, the rectal tolerance to these high radiation dose levels represents a significant clinical concern,” said Michael J. Zelefsky, M.D., Memorial Sloan Kettering Cancer Center. “The concept of an absorbable hydrogel implant pushing the rectum away from the prostate is a totally new approach and a potential game changer. The use of SpaceOAR for this clinical setting may reduce long-term rectal toxicity and become a critical adjunct to the safe implementation of high-dose SBRT [stereotactic body radiation therapy].”
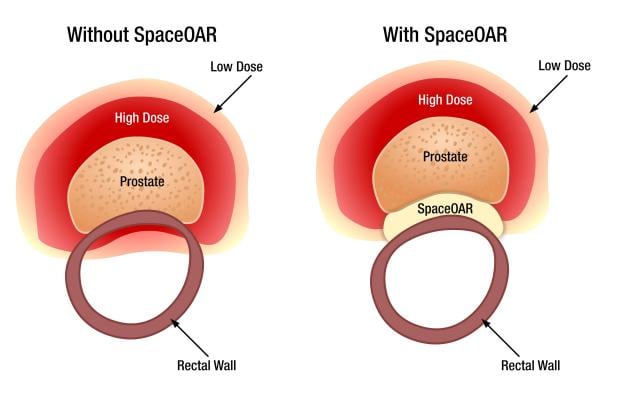
Despite advancements in prostate cancer radiotherapy, the close proximity of the prostate to the rectum (defined as the organ at risk, or OAR) limits the total amount of radiation that can be delivered safely to the prostate without fear of rectum radiation injury. In fact, in 1996 and 2003, the published median volume of rectum receiving 70Gy radiation (or V70, a predictor of long-term rectal complications) was 21.7 percent and 18.2 percent, respectively (RTOG 9406 and 0126 studies). In comparison, the median rectum V70 radiation in the recently published SpaceOAR study was 2.3 percent.
The low rectal radiation doses made possible by the SpaceOAR System will enable advanced prostate cancer radiation protocols that should result in fewer rectal complications (including rectal pain during treatment and declines in bowel quality of life), higher prostate radiation doses and faster treatment times, translating into better patient outcomes, reduced cancer recurrence and healthcare savings. These benefits are helping to drive rapid adoption of the technology for use in SBRT, intensity modulated radiation therapy (IMRT), low-dose rate brachytherapy (LDR), high-dose rate brachytherapy (HDR) and proton radiotherapy procedures.
The SpaceOAR System was the subject of several symposium presentations at ASTRO 2015, including:
- John Sylvester, M.D., radiation oncologist at 21st Century Oncology, presented the SpaceOAR U.S. Pivotal study results;
- Marcio Fagundes, M.D., medical director at Provision Center for Proton Therapy, discussed spacer use in proton radiotherapy; and
- Robert Timmerman, M.D., professor and vice chair of the Department of Radiation Oncology and professor of neurosurgery at UT Southwestern, discussed spacer use in dose-escalated SBRT.
Using a minimally invasive procedure, the SpaceOAR System is injected as a liquid into the space between the prostate and rectum where it expands the space and then solidifies into a soft hydrogel. The hydrogel remains stable for three months while protecting the rectum during radiotherapy and then liquefies and is completely absorbed.
The SpaceOAR System is FDA-cleared, CE marked and TGA-approved.
For more information: www.augmenix.com


 February 04, 2026
February 04, 2026 









