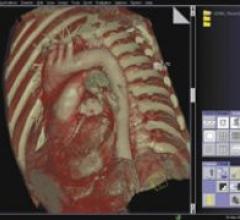
April 9, 2008 – CliniComp International, the clinical documentation tool used for more than 45 percent of all Department ...
The FDA gave market approval to the Medical Office Suite of Products, which aims to address the issue of coordination ...
The FDA cleared St. Jude Medical Inc.’s EnSite Fusion Registration Module, a software designed to help physicians create ...
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Based on Zone Sonography technology, the z.one ultra system now features advanced software, hardware and transducer ...
Heart disease is the leading cause of death of Americans, claiming hundreds of thousands of lives yearly. The ability to ...
A new line of functional and comfortable tracheostomy tube holders that are offered with designs for babies, kids and ...
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
Enova is releasing its new IRIS Solo 50 and IRIS Solo 100 Cordless Surgical Headlights in May of 2008. The Solo 50 ...
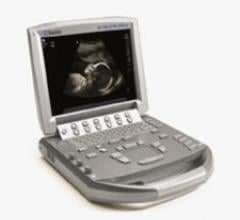
SonoSite Inc. recently released one of the first ultrasounds designed for musculoskeletal specialist. The S-MSK was made ...
Tethered to a hospital or healthcare system, one facility in a larger chain or just an independent medical provider in ...
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
The FDA 510(k) cleared the new Fuji DR (FDR) system, Unity SpeedSuite, a single detector designed to initiate image ...
The FDA gave Siemens Medical Solutions 510(k) market clearance of four new software applications used with the SOMATOM ...
Carestream Health showcased new capabilities for its Carestream RIS and PACS platforms. New features for Kodak ...
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
At AACN 2008, Vital Signs Inc. will display its new 500 and 1,000 mL InfusaScan fluid pressure infusor bags, which are designed to help reduce medication errors.
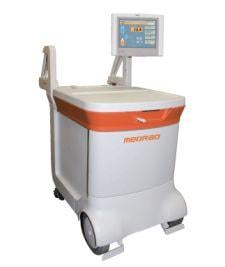
Medrad hopes to thrust new life into molecular imaging with its 510(k) pending FDG-PET power injector system called Intego, designed to promote greater accuracy in dose injection and which reportedly reduces radiation exposure related to FDG-handling by 40 percent.
April 9, 2008 – Saratoga Hospital in Saratoga Springs, NY, purchased IDC’s X2200, X1590 and X1600 direct digital ...
Integra LifeSciences Holdings Corp. introduced the next generation of MAYFIELD titanium skull pins designed for use with ...
Nucletron released three new products designed to improve brachytherapy practice. Designed for use in brachytherapy ...
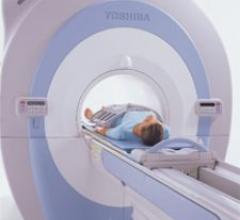
Toshiba America Medical Systems received FDA clearance for the new open-bore 1.5T Vantage Titan MR system, which will be ...
RIS/PACS solutions provider RADinfo Systems recently released PowerPACS DICOMmail software, which allows physicians to ...
SonoSite Inc. showcased its newly launched M-Turbo and S Series products, including the just released M-OB/GYN Office ...

 April 08, 2008
April 08, 2008