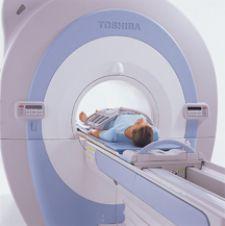
Toshiba America Medical Systems received FDA clearance for the new open-bore 1.5T Vantage Titan MR system, which will be commercially available in the first quarter of 2008.
The open bore of the Vantage Titan is 18 percent larger than other 1.5T systems on the market, featuring a large 71-centimeter patient aperture.
Toshiba says the Titan’s large clinical field-of-view is unique for this bore size and produces high-quality images without compromising homogeneity or overall imaging performance. The system also features Toshiba’s patented Pianissimo noise reduction technology, which creates a better imaging experience for all patients.
The Vantage Titan MR system also uses Toshiba’s proprietary, contrast-free MRA techniques – fresh blood imaging (FBI), contrast-free improved angiography (CIA), time-spatial labeling inversion pulse (Time-SLIP) and time-slip angiography (TSA). Contrast-free imaging is particularly important because gadolinium, the most common contrast agent used for magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) exams, has been directly linked to a sometimes fatal disease that occurs in patients with renal insufficiency, called nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD).


 February 13, 2026
February 13, 2026 









