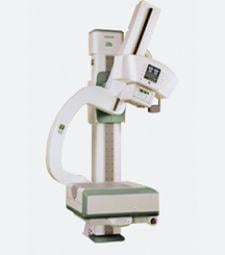
The FDA 510(k) cleared the new Fuji DR (FDR) system, Unity SpeedSuite, a single detector designed to initiate image display in two seconds, showing a fully processed image in seven to nine seconds.
Fully motorized, Unity SpeedSuite performs a variety of general radiographic exams, including both table-based and upright procedures. The system can reportedly be positioned and controlled to do almost any exam from chest to extremities, and is designed for any clinical area - general radiography, emergency departments and dedicated chest rooms or outpatient care facilities.
The new system features a single-button auto positioning and technique settings along with a Flash IIP technologist workstation that offers three-step processing and a common interface for Fuji CR and DR users. The system comes equipped with Harmonized Soft Copy (HSP) display parameters, allowing images to be presented to further enhance soft copy visualization when displayed at a PACS workstation.
Unity SpeedSuite includes a wireless hand-held remote and site-customizable settings, allowing users to program the exams that they perform most frequently. Said to require a smaller footprint than traditional DR systems, it provides an option for space-constrained rooms. Unity SpeedSuite also includes SpeedLink X-ray Control Software, an interface between the Flash IIP workstation and the X-ray generator. By automatically setting all exposure settings for each exam according to FUJIFILM’s anatomical menu parameters, SpeedLink eliminates workflow steps and saves time.


 August 14, 2025
August 14, 2025