Eizo Inc. recently announced that it received U.S. Food and Drug Administration (FDA) 510(k)…
Technology
Search by Company Name, Product Category, Product Name or by any combination of the three using the search boxes below.
Hitachi Medical Systems America Inc. announced its All-Inclusive Service and Support Program for…
May 2, 2016 — Elekta announced that Monaco version 5.11, the latest update to its…
Bayer announced that the U.S. Food and Drug Administration (FDA) has approved Gadavist (…
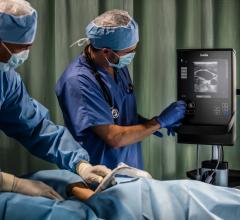
Konica Minolta introduced the latest version of the Sonimage HS1 compact ultrasound system,…
April 27, 2016 — IBA (Ion Beam Applications S.A.) announced the release of Dolphin Online Ready…
Philips announced that any hospital or healthcare facility with one of its indicated computed…
Siemens Healthcare announced the U.S. Food and Drug Administration (FDA) has cleared two new…
EOS imaging, a in 2D/3D orthopedic medical imaging, announced today that the U.S. Food and Drug…
Vital Images, Inc., a Minneapolis-based advanced medical imaging and informatics company,…
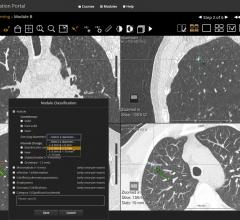
HealthMyne, an imaging informatics company, announced 510(k) clearance from the U.S. Food and…
Hologic Inc. announced the U.S. Food and Drug Administration (FDA) clearance and commercial…
Ikonopedia announced the completion of its first completely remote installation of its…
The 6D-Robotic Couch by gKteso ensures patient-positioning of sub-millimeter precision. The…
Fujifilm SonoSite Inc., received its CE mark and 510(k) clearance for the new mountable…
Toshiba America Medical Systems Inc.’s Aquilion Lightning computed tomography (CT) system was U.…
Dune Medical Devices has received pre-market approval (PMA) from the U.S. Food and Drug…
March 30, 2016 — Philips Healthcare introduced the first commerci
Carestream Health has filed a 510(k) application with the U.S. Food and Drug Administration (FDA…
Olea Medical announced U.S. Food and Drug Administration (FDA) clearance of Olea Sphere 3.0…


 May 04, 2016
May 04, 2016