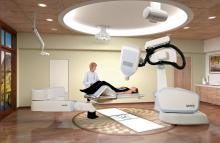
Accuray Inc. announced that the European CyberKnife Center Munich-Grosshadern (ECZM) has treated the world’s first patient with the new CyberKnife M6 System. With the installation of the new system, the Munich center, in close cooperation with the University Hospital of Munich, is the first to offer cancer patients treatment with the CyberKnife M6 System, the latest generation of the CyberKnife System. The new system is now able to provide enhanced quality, a streamlined user interface for treatment delivery and precision to radiation therapy treatments and continues to provide clinical capabilities including non-isocentric, non-coplanar robotic beam delivery and real-time tracking and automatic correction.