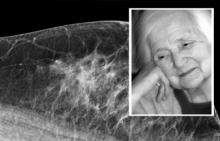
Intelerad Medical Systems launched its new Tomosynthesis Module. Integrated directly into Intelerad’s InteleViewer, the module eliminates the need for dedicated workstations, allowing practices to easily open new revenue streams, while enhancing radiologists’ workflows by allowing them to read tomosynthesis studies from their main workstation.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now