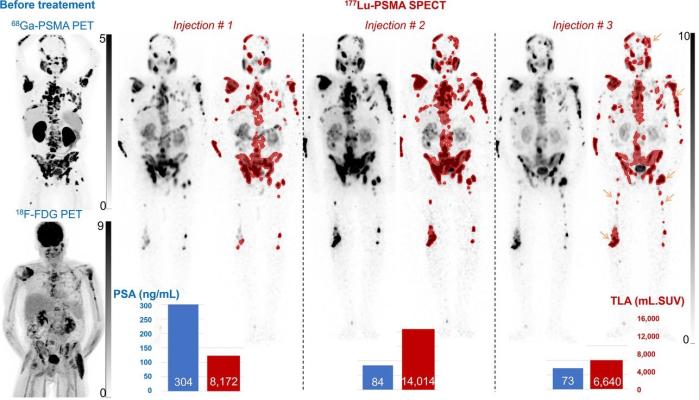
Maximum-intensity projection images from [68Ga]Ga-PSMA and [18F]-FDG PET before treatment, and [177Lu]Lu SPECT displayed without and with (red) segmentation with SUV of >3 in 74-y-old mCRPC patient. [177Lu]Lu-PSMA therapy was stopped at third injection despite dramatic decrease in PSA because of lack of favorable impact on symptoms and worsening clinical condition, and patient died 6 months later. High TLA was observed after second and third injections with advent of new bone lesions (orange arrows), both being indicative of poor prognosis. (Photo: The Journal of Nuclear Medicine)
Sept. 26, 2025 — A new fast and convenient approach to scintigraphy-based monitoring allows physicians to efficiently and reliably assess prostate cancer progression or regression during treatment. With this strong prognostic information, treatments for prostate cancer patients can be personalized according to tumor evolution, significantly impacting their overall survival. This research was published online in The Journal of Nuclear Medicine.
177Lu-PSMA is a highly-targeted radionuclide treatment for metastatic castration-resistant prostate cancer (mCRPC). Conventional monitoring techniques during 177Lu-PSMA therapy include analysis of the clinical condition and prostate-specific antigen (PSA) measurements. PSMA PET/CT is also performed before and during treatment. 177Lu SPECT imaging after each injection has shown potential for treatment monitoring, however, it often has long imaging times.
“This drawback makes it challenging to systematically perform SPECT after each 177Lu-PSMA injection — including after the last injection, which may provide the most substantial prognostic information,” said Laetitia Imbert, PhD, a physicist at Regional University Hospital Center in Nancy, France. “However, this technical issue can now be largely overcome with the advent of high-sensitivity 360° cadmium–zinc–telluride (CZT) SPECT systems, which enable total-body SPECT recordings in under 20 minutes. Our study sought to explore this new SPECT approach further in regard to 177Lu PSMA treatment monitoring.”
The retrospective study included 72 mCRPC patients who received up to six 177Lu-PSMA treatments. All patients underwent 68Ga-PSMA-11 PET before initial treatment, had their PSA measured before each injection, and underwent 177Lu-PSMA CZT SPECT after each injection. Quantitative image analysis and statistical image analysis were performed to predict overall survival.
Most PSA, PET, and CZT SPECT variables were significant univariate predictors of overall survival. However, only two 177Lu CZT SPECT variables were multivariate predictors: detection of new bone lesions during treatment and final total lesion activity (TLA). Means of survival times were 19.7 months in the 19 patients who showed no new bone lesions and a low level of TLA; 14.4 months in the 19 patients with only one of these two criteria; and 6.9 months in the 19 patients with neither criterion. “Fighting cancer is a battle against time, as the disease can evolve rapidly. This new scintigraphy camera will assist in changing treatment plans for patients who do not respond well, at the earliest stage possible,” noted Caroline Boursier, MD, a physician at Regional University Hospital Center in Nancy, France. “Improvements could be achieved by adjusting the injected
activity of 177Lu-PSMA, substituting this radionuclide therapeutic agent with an alternative, or combining it with other cancer treatments such as chemotherapy, external radiotherapy, immunotherapy, or anti-angiogenic drugs.”
She added, “Logistically, monitoring patients with CZT SPECT is easy to schedule because of the very fast imaging times and the fact that no additional tracer injection is required. Scintigraphy imaging can therefore begin as soon as the patient arrives at the nuclear medicine department, and in our experience, they can leave in less than 30 minutes.”
The authors of “Prognostic Value of Comprehensive Analysis of Metastatic Prostate Tumor Changes from First to Last [177Lu]Lu-PSMA Therapy Injections Through Serial High-Speed Whole-Body 360° Cadmium–Zinc–Telluride SPECT” include Julien Kunsch, Pierre Olivier, and Marine Claudin, Department of Nuclear Medicine, CHRU Nancy, Nancyclotep Imaging Platform, Nancy, France; Timothée Zaragori, Innovation Technologique, CIC 1433, CHRU-Nancy, INSERM, Université de Lorraine, Nancy, France, and IADI, INSERM, U1254, Université de Lorraine, Nancy, France; Pierre-Yves Marie, Sébastien Heyer, Antoine Verger, Laetitia Imbert, and Caroline Boursier, Department of Nuclear Medicine, CHRU Nancy, Nancyclotep Imaging Platform, Nancy, France, and IADI, INSERM, U1254, Université de Lorraine, Nancy, France; and Perrine Raymond, Department of Endocrinology, CHRU Nancy, Nancy, France.
Visit the JNM website for the latest research.


 February 26, 2026
February 26, 2026 









