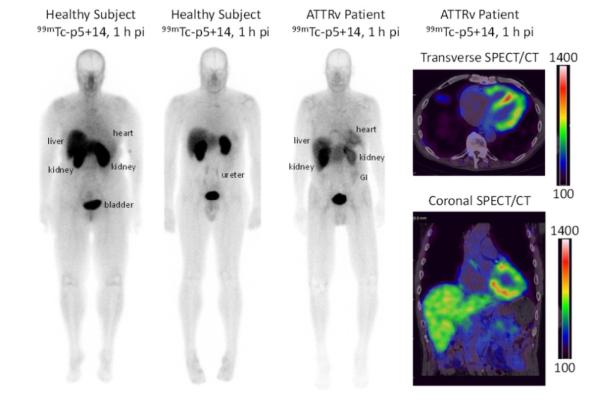
Figure 1. Representative whole body planar images of 99mTc-p5+14 of healthy subjects and, with SPECT/CT images, of a patient with ATTRv cardiac amyloidosis at 1 h post injection showing no uptake in the heart of the healthy subject and intense signal in the heart of the patient using both planar and SPECT/CT imaging.
Abstract 241277. “Preliminary Evaluation of 99mTc-Labeled Peptide p5+14 for the Detection of Cardiopulmonary Amyloidosis Using SPECT/CT and Planar Gamma Scintigraphic Imaging,” Jonathan Wall, Emily Martin, Alan Stuckey, Bryan Whittle, Joseph Jackson, Angela Williams, Trevor Hancock, R. Eric Heidel, Muddassir Mehmood, Anne Kassira, Ronald Lands, Hannah Watson, Rebecca Hung, Stephen Kennel, University of Tennessee Graduate School of Medicine, Knoxville, Tennessee.
June 11, 2024 — A newly developed radiotracer can generate high quality and readily interpretable images of cardiac amyloidosis, a condition referred to as the “Alzheimer’s disease of the heart.” As the first amyloid-specific and pan-amyloid binding radiotracer designed for planar and SPECT/CT imaging, 99mTc-p5+14 could play an important role in early detection and treatment of cardiac amyloidosis. This research was presented at the 2024 Society of Nuclear Medicine and Molecular Imaging Annual Meeting.
Systemic amyloidosis is an incurable disease in which abnormal amounts of proteins build up within the body’s tissues and organs. About 20 percent of patients who have amyloid build-up in the heart experience early deaths. While recent progress in the treatment of cardiac amyloidosis has greatly improved patient prognosis, median survival remains low at approximately three to five years.
“Therapies that slow the progression of amyloid deposition have been developed; however, they are not effective in patients with late-stage disease. Therefore, the ability to detect cardiac amyloidosis early is critical,” noted Jonathan Wall, PhD, director of the Amyloidosis and Cancer Theranostics Program and professor at the University of Tennessee Graduate School of Medicine in Knoxville, Tennessee. “Unfortunately, there are currently no Food and Drug Administration (FDA)-approved imaging agents that detect cardiac amyloidosis.”
To address this issue, researchers developed a novel technetium-99m labeled variant of the pan-amyloid reactive peptide p5+14 (99mTc-p5+14). In the first-in-human study, five healthy volunteers and 30 patients newly diagnosed with light chain or transthyretin amyloidosis underwent 99mTc-p5+14 imaging with standard planar gamma scintigraphy and SPECT/CT. Blood was collected to assess serum biomarkers, and a transthoracic echocardiogram was performed. Standard 99mTc-pyrophosphate imaging was also performed on most patients at 72 hours after 99mTc-p5+14 image acquisition.
The planar and SPECT/CT images generated using 99mTc-p5+14 were of high quality and readily interpretable at both one and three hours post-injection. Patients with amyloid cardiomyopathy had significant 99mTc-p5+14 uptake in the heart, whereas no cardiac uptake was observed in healthy subjects.
“Early and accurate diagnosis of cardiac amyloidosis is crucial to ensure the most positive outcomes for patients,” said Wall. “Imaging with 99mTc-p5+14 could provide an easy to use and interpret technology that could be employed in the community cardiology setting, where SPECT imaging is common, as a rapid screen for amyloid cardiomyopathy in the future.”
The 99mTc-p5+14 radiotracer is currently in ongoing early-stage clinical evaluation at the University of Tennessee Graduate School of Medicine in conjunction with Attralus Inc., to assess safety and efficacy in patients with cardiac amyloidosis and healthy subjects. The data and insights obtained from this research will support initiation of a pivotal Phase 3 study and approval submissions to the FDA in the coming years.
For more information: www.snmmi.org


 March 06, 2026
March 06, 2026 









