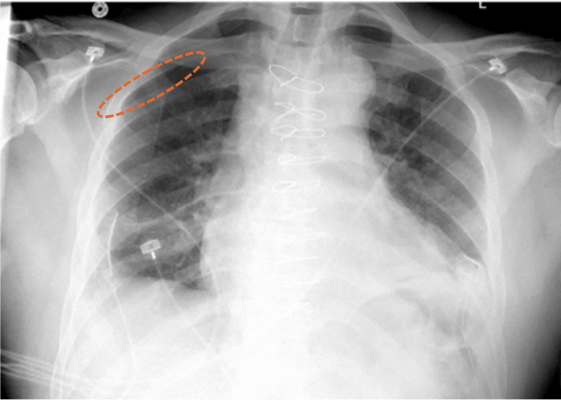
March 31, 2022 — Aidoc, a leading provider of healthcare AI solutions, announced that it received FDA 510(k) clearance for its triage and notification of pneumothorax on X-ray exams. A one-stop partner for the enterprise’s clinical AI needs, Aidoc’s other seven FDA-cleared solutions are already implemented across U.S. health systems, flagging and communicating suspected pathologies in CT exams – and now have expanded to the high volume X-ray modality.
Aidoc’s newly FDA-cleared solution runs on all X-ray machines including portable ones, and is designed to analyze X-ray images. It automatically flags positive cases of pneumothorax, facilitating physicians to read X-rays in a timely manner.
The ability to quickly identify pneumothorax is imperative as it can worsen rapidly and result in respiratory or cardiac failure. This however is easier said than done, due to the extensive imaging volumes radiologists have to continuously examine. Aidoc’s highly accurate AI pneumothorax algorithm addresses this challenge by analyzing X-rays, flagging, and notifying physicians of the suspected findings.
“We’re very excited about this important milestone,” said Elad Walach, CEO of Aidoc. “This FDA clearance further validates the breadth of our AI platform, going beyond specific AI algorithms to act as a healthcare AI hub for the enterprise’s cross-specialty needs. This includes ER, ICU, outpatient centers, inpatient admissions, and the coordination of care and communication among providers. By bringing radiologists and proceduralists to the same AI platform, we enable enhanced collaboration across departments and systems to deliver patients with the right treatment at the right time.”
Aidoc’s AI platform processes high volumes of scans and images without disrupting the physician’s workflow. Existing partners of Aidoc, who add the pneumothorax solution to their portfolio, will not require any additional integration, infrastructure, or maintenance efforts.
For more information: www.aidoc.com


 February 13, 2026
February 13, 2026 









